Alzheimer’s Disease (AD) is a type of dementia which induces progressive and non-reversible brain disorders (memory and cognitive loss, thinking and behavior changes…). AD ultimately ends in severe mental dysfunction due to neuronal death and breakdown of neural connections. The majority of people with AD are 65+ (late-onset AD). Even if age is one of the greatest risk factors, >5% of AD patients are in their 40s or 50s (early onset AD) making genes and heredityother important AD risk factors (1-4). The main hallmarks in AD brains are two abnormal structures: neurofibrillary tangles and amyloid plaques. These structures are issued from the self-assembly and deposits of molecules. While neurofibrillary tangles consists of twisted fibers of the phosphorylated tau protein inside cells, Amyloid plaques are the results of the accumulation of Amyloid Beta peptide (Aß) to form ß-amyloid complexes (Aß, 1-42) in between nerve cells. The Aß is derived from the cleavage of the Amyloid Precursor Protein (APP); its a length varying between 39 to 43 amino acids.
A urgent need for immuno-assays & histological stains highly specific to Aß
Even if the origin of ß-amyloid complexes is beginning to be understood, their roles in AD and dementia are yet unknown. Researchers think they might be involved in the loss of communication between neural cells and in their progressive death occuring during AD. The neurotoxicity of intraneuronal Aß accumulation is an area of considerable research. Nevertheless some experimental hurdles can be met when using antibodies or histological stains (lack of specificity, cross-reaction, weak labelling, stability…). Immuno-assays are valuable tools to detect Aß complexes in human CSF and brain tissue extracts (qualitative approach by WB, quantification by ELISA), but only if the antibody used is specific for Aß and does not detect APP. As for histological stains, they need to have optimised chemical characteristics for improved Aß detection in tissue sections.
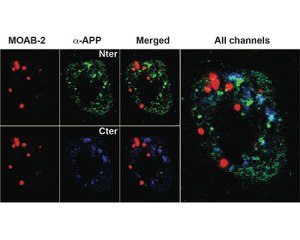
MOAB2 a new high-titer and highly Aß specific monoclonal
MOAB-2 (mouse IgG2b) antibody, developed by LaDu and co-workers (2) and available through Biosensis and tebu-bio, has been shown to specifically detect Aß, but not the precursor molecule APP. MOAB-2 detects preparations enriched in U-, O-, F-Aß42, and U-Aß40 by dot-blot, and is thus a pan-specific Aß antibody. However, MOAB-2 is selective for the more neurotoxic Aß42 compared to Aß40. Indeed, MOAB-2 demonstrated a titration against antigen concentration, and detects Aß40 at 2.5 pmol but U-, O- and FAßb42 at antigen concentrations as low as ~ 0.1 pmol. MOAB-2 demonstrates strong intraneuronal and extra-cellular immunoreactivity in 5xFAD and 3xTg mouse brain tissues. MOAB2 is guaranteed for IP, WB, IHC, IHC(P), IF. When used in ELISA, the oligomeric form of Aß peptide (o-Aß) can be assayed independently of the other forms of the molecule.
Ready-to-use sandwich ELISA Kit specific for Oligomeric Aß using MOAB2 antibody
This o-Aß ELISA kit (an exclusivity from Biosensis – tebu-bio) is a sandwich ELISA that allows the quantification of oligomeric Aß peptides (3, 4).
Ready-to-use, the kit consists of a pre-coated mouse monoclonal MOAB-2 anti-Aß capture antibody, a biotinylated MOAB-2 detection antibody and horseradish peroxidase (HRP)-conjugated streptavidin and TMB substrate.
The readout obtained yields a colored reaction product which is directly proportional to the concentration of o-Aß present in samples and protein standards.
The inclusion of a highly validated oligomeric standard in the kit makes it ideal for in vitro qualitative measurement of o-Aß peptide levels in brain extracts and CSF samples from both transgenic mice and humans relative for research and with unique characteristics:
- Range: 15.6 – 1000 pg/mL (o-Aß standard curve: 10-1000 pg/mL)
- Sensitivity: 30-60 pg/mL (defined as 150% of blank value)
Bright blue UV excitable stain for histological localization of Aß plaques
Traditional methods for the histological localization of Aß plaques rely on the use Congo Red or Thioflavin S. However, these conventional stains display sub-optimal spectral characteristics and limited uses for ideal tissue section analysis of Aß plaques. Amylo-Glo® RTD™ is a bright blue UV excitable ready-to-dilute reagent to stain Aß plaques in tissue sections. Bright, Amylo-Glo® RTD™ is ideal for low magnification quantification studies. With an excitation peak at 334 and an emission peak at 533 nm (438 nm when bound to amyloid) and mild staining conditions), Amylo-Glo® RTD™ can be used in combination with multiple immunofluorescent labeling studies. Compatible with with fresh, frozen, and formalin-fixed immunohistochemistry or cytochemistry, this unique stain is well-suited for confocal and multiple labeling because of its high fluorescent intensity and high resistance to photo-bleaching. Moreover because Amylo-Glo® fluoresces in the UV channel, double and triple labeling experiments can be performed very easily.
Amylo-Glo® RTD™ simple 5-step staining protocol:
- Dried, prepared slides are transferred into a 70% solution of ethanol for 5 minutes at room temperature,
- The slides are then rinsed in distilled water for 2 minutes, without shaking.
- The slides are then incubated for 10 minutes in the prepared 1X (A-*57′? staining solution.
- The slides are then rinsed in 0.9% saline solution for 5 minutes, without shaking.
- The slides are then rinsed very briefly in fresh distilled water (DW), approximately 15 seconds.
Note: a kit with EtBr counter staining of nuclei and cell bodies for easy identification and spacial orientation also exists.
Want to know more about Aß research tools from tebu-bio?
tebu-bio provides a comprehensive range of reagents dedicated to AD and neurobiology studies. The ones described in this post are the following:
- MOAB-2 (mouse IgG2b) antibody to specifically detect Aß.
- o-Aß ELISA kit to quantify oligomeric Aß peptides.
- Amylo-Glo® RTD™ to stain Aß plaques in tissue sections.
Contact us if you’d like to benefit from even more innovative research solutions in neurobiology! [contact-form to=’europe@tebu-bio.com’ subject=’AD and Amyloid beta research tools’][contact-field label=’Name’ type=’name’ required=’1’/][contact-field label=’Email’ type=’email’ required=’1’/][contact-field label=’Please add your comment’ type=’textarea’ required=’1’/][/contact-form] Sources:
- Tai L. M. et al. “Soluble apoE/Aß complex: mechanism and therapeutic target for APOE4-induced AD risk” (2014) Molecul. Neurodegener., vol. 9:2. DOI: 10.1186/1750-1326-9-2
- Youmans K.L. et al. “Amyloid-ß42 alters apolipoprotein E solubility in brains of mice with five familial AD mutations” (2011) J. Neurosci. Methods, vol. 196 no 1, 51-59. DOI: 10.1016/j.jneumeth.2010.12.025.
- Youmans K. L. et al. “Intraneuronal Aß detection in 5xFAD mice by a new Aß-specific antibody” (2012) Mol. Neurodegener, vol. 16, 7:8. DOI: 10.1186/1750-1326-7-8.
- Tai L.M. et al. “Levels of Soluble Apolipoprotein E/Amyloid-ß (Aß) Complex Are Reduced and Oligomeric Aß Increased with APOE4 and Alzheimer Disease in a Transgenic Mouse Model and Human Samples” (2013) J. Biol. Chem., Vol. 288 no 8, 5914–5926. DOI: 10.1074/jbc.M112.442103
- Schmued L. et al. “Introducing Amylo-Glo, a novel fluorescent amyloid specific histochemical tracer especially suited for multiple labeling and large scale quantification studies” (2012) Journal of Neuroscience Methods Vol. 209 no 1, 30 July 2012, 120–126. DOI: http://dx.doi.org/10.1016/j.jneumeth.2012.05.019



