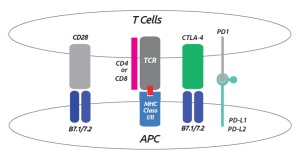
Activation of the immune system involves a number of checkpoints in order to ensure proper activation. For instance, T cell activation requires binding of the T cell receptor to the MHC antigen; yet this interaction alone is not sufficient for producing a T cell response. Full T cell activation and inactivation requires the coordination of a multitude of co-stimulatory and inhibitory signals.
For example, T cell activation can result from the combination of MHC:TCR and B7-1:CD28 interaction. In contrast, B7-1 binding to CTLA4 results in an inhibitory signal that prevents activation.
Given its diverse function and the fact that the immune system plays a role in virtually all human diseases, immunotherapy has become an important approach for the treatment of numerous diseases.
Immunotherapy in drug discovery programs

Immunotherapy is defined as the treatment of disease by inducing, enhancing, or suppressing an immune response.
This often involves targeting immunoreceptors, such as those discussed above, with small molecule inhibitors/activators or immune system components, including antibodies or antibody fragments (Fab). Immunotherapies have shown efficacy in the treatment of cancer, autoimmune diseases, inflammatory diseases, and more.
Pathway-specific Immunotherapy screening biochemical assays

To enable researchers and drug discovery screeners to run their experiments with optimized high quality kits, many efforts in developing proteins, antibodies and ready-to-use kits have been made. I made a selection of some of them in my previous posts. A few examples related to specific pathways from BPS Biosciences:
- Immunotherapy Screening – IDO pathway
- Immunotherapy Screening – CD137:CD137L pathway
- Immunotherapy Screening – CD40:CD40L pathway
- Immunotherapy Screening – GITR:GITRL pathway
- Immunotherapy – BLTA:HVEM, CD47:SIRPα in drug discovery
- Immunotherapy Screening – A focus on PD-1/PD-L1/PD-L2 Pathway in drug discovery
- Immunotherapy Screening – B7-1 / CD28 and B7-1 / CTLA4 Pathways in Drug Discovery
A cell-based assay to assess IDO1 and TDO
L-tryptophan (L-Trp) is an essential amino acid for protein synthesis in mammalian cells. Its catabolism to Kynurenine (Kyn) is defined as a key regulator of innate and adaptive immunity. This L-Trp to Kyn catabolism maintains an immunosuppressive microenvironment by starving immune cells of L-Trp and releasing degradation products of LTrp that possess immuno-suppressive functions.
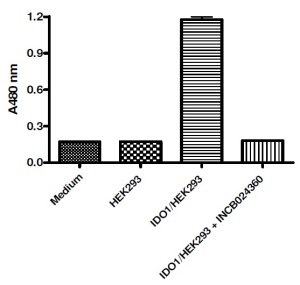
Indoleamine 2,3-dioxygenases (IDO1 & IDO2) as well as Tryptophan 2,3 dioxygenase (TDO) have key regulatory roles in this pathway. They are upregulated in many tumors, providing cancer cells with an avenue for immune evasion.
The human IDO-1 Cell Based Assay Kit and TDO Cell-Based Assay Kit are designed to monitor the activity of exogenously expressed human IDO1 or TDO in cultured cells.
The kits contain a “transfection-ready” expression vector for full length human IDO1 (hIDO1) or TDO (hTDO), respectively. Upon expression, hIDO1 or hTDO can catalyze L-Trp conversion to Kyn, which gets released and is easily detected in the assay medium. The kits also include specialized assay media and all components necessary to fully activate hIDO1 or hTDO.
Would you like further information about Immunotherapy related proteins and screening kits?
Just get in touch through the form below.