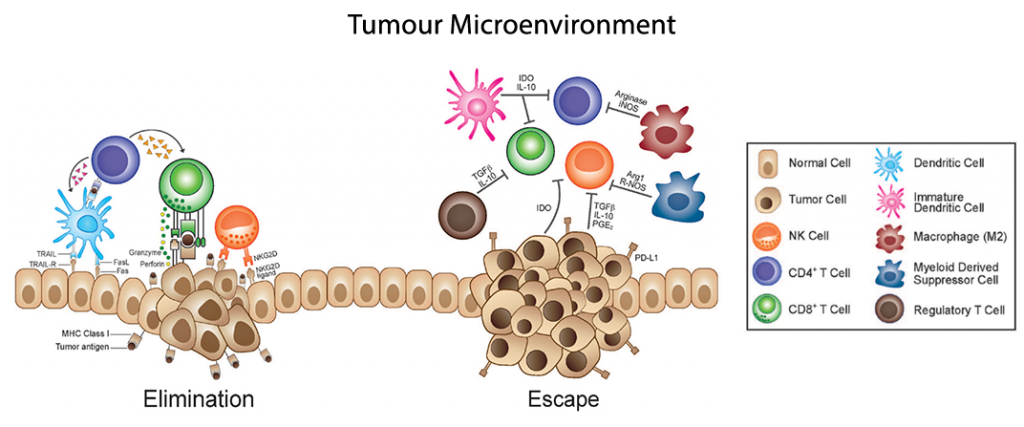
Immunoediting of cytokine signals provides another area of pathways which tumours utilise to evade and potentially escape from immunological targeting. This post aims at shedding some light into the Tumour Microenvironment (TME) and particularly on how the tumour recruits accomplices to grow and disseminate.
Tumour immunosurveillance crosstalks – the main actors to monitor
TGF-β, VEGF, angiogenic chemokines, and T helper 2 polarising cytokines form a nexus of signals that promote tumourigenesis in certain settings. While TGF-β may indeed induce cell cycle arrest, it has many different effects within the TME. Primed CD8 T cells receiving TGF-β signaling can block priming into fully mature CTLs, and also drive the differentiation of CD4 T cells towards a tumour-supporting T-helper 2 phenotype (Th2) or regulatory T cell population (Treg), rather than a more anti-tumor Th1 phenotype.
This CD4 Th2 phenotype is characterised mainly by expression of IL-4, IL-5, IL-6 and IL-10, opposite the inflammatory and anti-tumour phenotype of Th1 CD4 T cells expressing IFN-γ, TNF-α, and IL-2. Th2 polarisation within the TME is further promoted by the expression of IL-10, TGF-β, and VEGF, which facilitates recruitment and differentiation of myeloid-derived suppressor cells (MDSCs), a myeloid progenitor elevated in virtually all experimental malignancies.
MDSCs promote the TME in response to TGF-β signaling by expressing multiple angiogenic chemokines including CXCL-1, -2,-3, -5, -8, and -12 (amongst others), helping provide continued sustenance to the growing tumour. Increased TGF-β signaling, resulting in the conversion of CD4 T cells into a TREG population may promote further immunosuppressive functions, though their potential role(s) in tumourigenesis remains to be determined.
VEGF is an additional pleiotropic cytokine produced inside the TME, and possesses potent immunosuppressive, inflammatory and proangiogenic capacities. The hypoxic nature of the TME results in increased HIF-1α expression which leads to increased inflammatory conditions characterised by TNFα, IL-6, and IL-8. Conveniently, these same inflammatory factors promote elevated levels of VEGF, resulting in a feedback loop of VEGF signaling, further promoting a favourable TME. VEGF is also a chemoattractant for macrophages (Mφs), and with other signals surrounding the TME, these Mφs are often further primed into the MDSC population, which feeds back to promotes the immunosuppressive nature of the TME, and facilitates tumour immunosurveillance evasion.
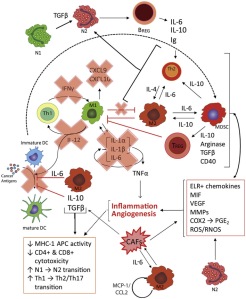
In the TME, there exists a positive feedback loop of cytokine signals that proceeds as follows.
- First, TGF-β, COX2, PGE2, Th2- associated inflammatory factors and proangiogenic proteins are secreted by cancer cells, CAFs and other cell types in the nascent tumour recruit Th2 lymphocytes, M2 macrophages (TAMs) andN2 neutrophils (TANs).
- Then, Th2 lymphocytes, TAMs and TANs secrete additional inflammatory and proangiogenic proteins that suppress maturation of DCs and proliferation and activation of cytotoxic cells.
- As a result, antigen presentation and cytotoxic activities plummet, practically eliminating immunosurveillance in the tumour milieu. Additionally, B cells proliferate, but are not activated, turning them into tumour-promoting BREGs. M2 macrophages recruit MDSCs to the TME, further reinforcing the positive feedback loop of Th2, M2, and N2 proliferation and activation, resulting in substantial increases in tumour-promoting inflammation and concomitant angiogenesis.

L-series arrays allow to study up to 1,000 biomarkers in a biological sample, simultaneously.
Antibody Arrays for Cancer Biomarker Discovery
Interrogation of the tumour environment’s niche of cell signals, growth factors, and cytokines, as well as the TME recruitment of accessory cell population and their cytokines, requires a global view of all these factors together. While a piecemeal approach (e.g. using individual ELISAs) may prove effective if the hypothesis is sufficiently narrow, a broad view of cancer will be needed for biomarker discovery and diagnostics moving forward.
We believe that a type of “immunoscoring” may be warranted, whereby we can monitor the changes in a patient’s immune response, either globally or within the TME, as a routine measure of efficacy, prognosis, or disease state for cancer patients. Such a score would be heavily facilitated by the use of antibody arrays which allow for upwards of 1,000 proteins to be measured from any sample. The multiplex platform may reveal new cytokine interactions, define new complex growth factor pathways or angiogenesis mechanisms that could help open the doors to new techniques or strategies in battling this worldwide battle against cancer.
Looking for validated immunoassays to analyse tumour crosstalks?
Interested in unraveling the mechanisms of TME and immunosurveillance in your cancer patients or cancer experimental model? Leave a message below.