In this post, I’d like to introduce the Human liver Lysosomes developed by Sekisui-Xenotech, which have opened up a new era in catabolism models. Read on to learn more, and also to download a case study demonstrating the use of lysosomes for catabolism of human IgG.
Lysosomes are membrane-bound cellular organelles. They are the site of degradation/catabolism, including extracellular substrates (endocytic pathway) and intracellular substrates (autophagy pathway). Lysosomes contain a multitude of acidic hydrolytic enzymes. They vary greatly in size and have Isopycnic densities similar to mitochondria.
Considering their unique pH and the presence of a variety of catabolic enzymes, therapeutic strategies are being designed to take advantage of lysosomes as the primary site of catabolism/activation for targeted biopharmaceuticals that enter cells through the endosomal-lysosomal pathway.
Why use Human Liver Lysosomes in in vitro drug development?
Sekisui XenoTech’s Human Liver Lysosomes provide the most high-quality, specific, relevant, easy and ready to use in vitro test system to monitor stability/catabolism of targeted biotherapeutics. Purified recombinant enzymes, cell homogenates or cell lines engineered to overexpress certain proteins cannot replicate the environment that compounds will encounter in the native lysosome, nor do they take into account inherent differences between primary human tissue and cell lines.

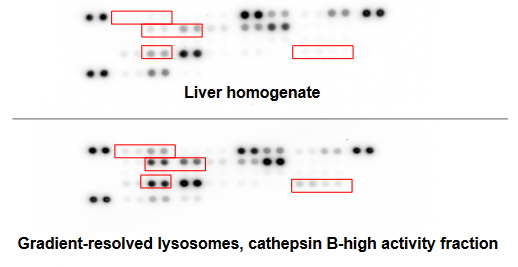
Purified hepatic lysosomes offer a complete and stoichiometrically correct enrichment of lysosomal contents from human primary tissue for greater lysosomal enzymatic activity and enrichment of lysosomal proteins with low contaminating activity from mitochondrial enzymes. The lysosomal isolation protocol used has been optimized through characterization by immunoblot, enzymatic activity and protease content.
Applications of the Human Liver Lysosomes
Lysosomes can be used as an in vitro diagnostic tool to conveniently, cost-effectively and quickly evaluate potential changes in lysosomal stability due to targeted modifications of the biopharmaceutical/macromolecule during development. The data can help narrow and direct development tracks of biopharmaceuticals such as those interested in:
- Antibody-Drug-Conjugates (ADCs)
- siRNA/RNAi technologies
- immunotherapies
- biodegradable copolymers and nanoparticles as delivery mechanisms
- cosmetics using microparticles etc.
Case study with Human Liver Lysosomes: Functional characterization – catabolism of human IgG
Also available: Rat Tritosomes

Tritosomes are lysosomes isolated from Tyloxapol-treated animals for improved separation of the organelle from the mitochondria.
- Cathepsin L Human ELISA Kit – cat. nr 126ELH-CathepsinL
- Cathepsin S Human ELISA Kit – cat. nr 126ELH-CathepsinS
- Cathepsin B Activity Assay Kit – 12668AT-CathB-S100
- Acid Phosphatase Activity Colorimetric Activity Assay Kit – 12668CL-AcPh-S500
- Anti LAMP-1 / CD107a polyclonal antibody – cat. nr 281BS6978
- Anti CTSD monoclonal antibody (M01), clone 3F12-1B9 – cat. nr 157H00001509-M01
- Bafilomycin A1 | Vacuolar H+ ATPase inhibitor (CAS: 88899-55-2 – cat. nr 21910-2060)
Bafilomycin A1 (macrolide antibiotic) inhibits autophagy via disruption of fusion between autophagosomes and lysosomes.

2 responses
Is this a compatible testing environment for gcba inhumans to detect retention or toxicity levels?
Good Morning Reidun,
Thank you for your question on this post. I talked to our expert, Dr Chris Bohl from Sekisui Xenotech, to address it and I must say we do not have a clearcut answer on this. Here are his comments below. I hope you will find them helpful.
“MRI gadolinium containing contrast agents can cause transient and long term side effects when given to patients. It appears that with normal kidney function, the majority of it is excreted through the kidneys, however increasing data is indicating that small amounts of GCBA are being retained in various tissues and causing health problems in relatively healthy patients. There are at least 9 forms of GCBA approved for use in the US, each with their own unique properties and tissue retention profiles, although it appears that there are less approved for use in the EU (linear forms not approved). From what Chris gathered, toxicity is occurring when the GCBA retained in the tissue breaks down and releases “free” gadolinium, which then interferes/competes with Ca and interrupts Ca dependent biochemical reactions/pathways leading to health problems.
It seems like GCBA stability is assessed in vitro in aqueous solutions with various pHs and that this is only useful in predicting the in vivo thermodynamic stability and lability of the GCBA. If the GCBA is endocytosed and trafficked to the lysosome and is acted upon by a lysosomal enzyme which alters the GCBA stability/liability, then the isolated lysosomes may be useful in assessing stability and perhaps identifying metabolites of the GCBA, much like microsomes are used to asses stability/metabolites in the liver compared to stability in plasma. One parameter that needs more consideration is the incubation buffer composition. While Sekisui Xenotech has a catabolic buffer that can be used in conjunction with the isolated lysosomes, it is optimized more for proteolytic activities of the lysosome and this buffer may not be optimal for other lysosomal activities (ie. pH and redox state). While the scientific literature is in dispute about the internal environment of the lysosome in vivo, it is highly unlikely that a single buffer can truly replicate the dynamic environment of the intact, in vivo lysosome.
With regards to using the lysosome or Tritosomes for retention assays, Chris does not think that these will be very helpful as the lysosome structure has been disrupted by sonication. Perhaps the lysosomes could be used in assessing GCBA or metabolites for membrane binding, not sure that would be useful information but you never know!”
We hope this was useful anyway and remain available should you have anymore question.
Kind regards,
Isabelle (with Chris precious input!)