Understanding cancer cells’ structure is key to developing new therapies against it and preventing it. To do so, scientists can use a wide variety of technologies. One of them is the synchrotron.
A team of scientists at the University of Surrey have used the Diamond Light Source, a synchrotron in a science facility in Oxfordshire, to perform extensive in-depth analysis of the characteristics of cell structures at a nano level and even at an atomic scale.
They set out to learn more about how cells and materials interact with each other. Their endeavour could well open up new ways to detect cancers earlier and treat them better.
Why use a synchrotron for this research?
A synchrotron is a specific type of cyclic particle accelerator. Its ancestor is the cyclotron: a machine that accelerates charged particles outwards from the centre of a flat cylindrical vacuum chamber in a spiral (a magnetic field holds their path and an electric field accelerates them).
Use in nuclear medicine…
These machines are very helpful in nuclear medicine, a field that uses ingested radioactive substances to produce an image of the body thanks to radiation that comes “from within”. Contrary to x-ray imaging for instance, that uses radiation from outside the body to create an image of anatomy, whereas nuclear medicine uses this radiation to create an image of bodily functions.
When using the synchrotron, the experiments take place in what are called light lines. The magnetic field present in the apparatus folds the different particle bundles into a spiral synchronised (hence the name) to allow the acceleration of elementary particles to high energy.
The biggest synchrotron in existence is the Large Hadron Collider (LHC) in Geneva, Switzerland, built by the European Organisation for Nuclear Research (the CERN). It has a 27-kilometre circumference.
… And in x-ray imaging
Synchrotrons can also be extremely helpful in x-ray imaging, as the x-rays beams emitted by the electrons as they circulate around the synchrotron are different from those emitted by an imaging device. The very bright x-rays emitted by electrons in synchrotrons can yield more information as their properties are different from “normal” x-rays.
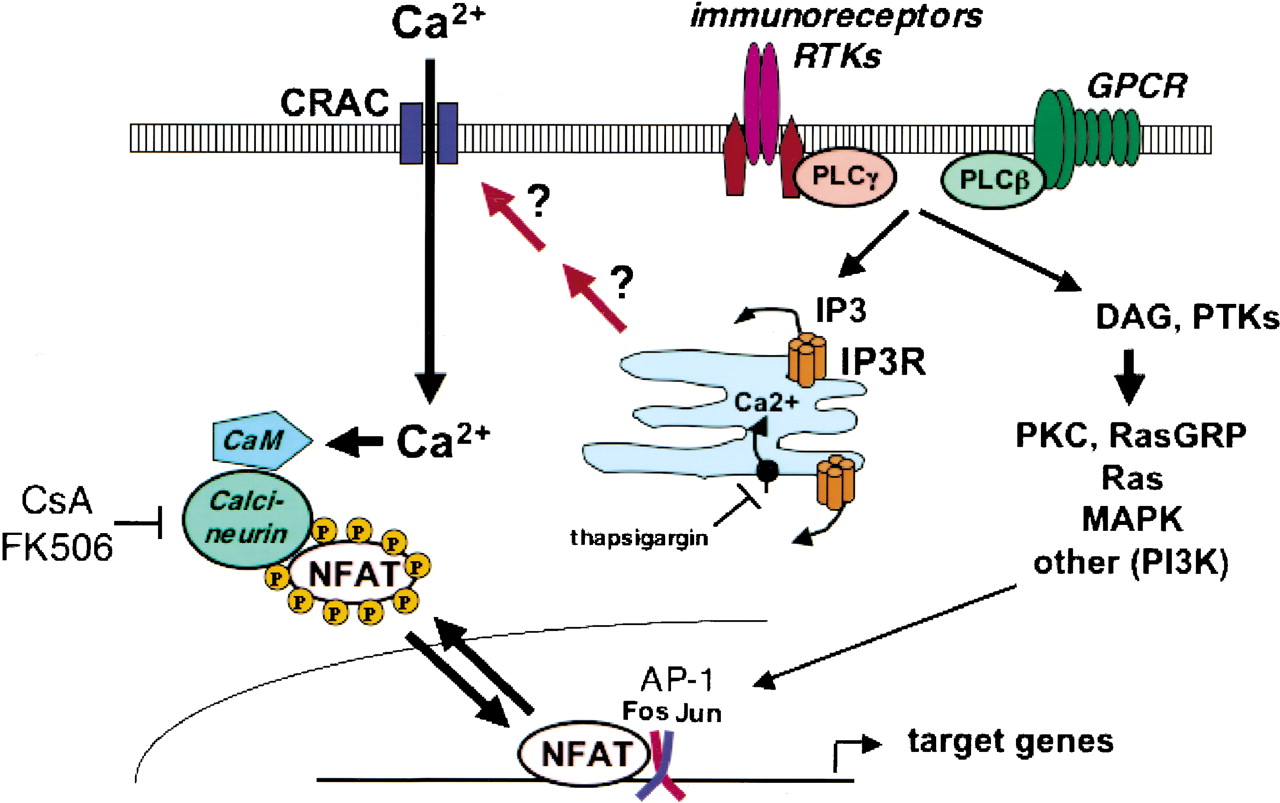
The two techniques used by the researchers to analyse cancer cells’ structural evolutions in our case are SAXS and WAXS: Synchrotron-based small- and wide-angle X-ray scattering. The researchers also combined synchrotron use with confocal laser scanning microscopy.
By observing the structural evolutions in pancreatic cancer, the researchers were able to obtain data that could eventually help develop better drugs/treatments and better understand cancer evolution.
What did the researchers actually do?
Complex examinations of cancer cells’ structure at the nano level (and even at an atomic scale) can yield crucial data on how cells and materials interact with each other.
This is why the team comprised of Jingyi Mo, Nathanael Leung, Priyanka Gupta, Bin Zhu, Hui Xing, Jiao Zhang, Eirini Velliou and Tan Sui from the University of Surrey (publishing in Journal of Materials Research and Technology and Materials Today Advances) used the Oxfordshire synchrotron.
Understanding cancer cells through 3D
Previous research had made significant headway in modelling pancreatic tumours accurately in 3D (including all its functions and features, especially concerning porosity, structure and protein composition).
This latest research experimented on a way to mechanically improve these 3D models to better understand how cells interact with each other and protein matrices at nanoscale.
Making these 3D structures and their mechanical performance in laboratory test conditions as close as possible to those of actual cancer tissue can produce better data for the scientific world.
The goal on the long term being of course to develop better treatments for pancreatic cancer – or, as the study refers to it, “pancreatic ductal adenocarcinoma”, as it affects the pancreatic duct by growing what is known as adenocarcinoma, a type of cancerous tumour that is an excessive growth of epithelial cells of glandular origin.
The researchers made models of the tumour in a lab (by freeze-casting polyurethane scaffolds, structures to hold the cells) and surfaced them with extracellular matrices to reproduce the cancer’s environment.
They then used the synchrotron’s x-rays and laser-scanning microscopy to analyse the evolutions of these structures and performed the same analyses while using different protein mixes (they also analysed uncoated structures so they could better understand all the interactions at play here).
They obtained tangible data on the deformation mechanics and mechanical properties of every part of the structure while observing them at different levels. On the use of mechanical engineering for their research, study leader Dr. Jingyi Mo commented that “combining advanced mechanical characterization of biomaterials with local scale cell behaviour [could open] doors to new scientific discoveries”.
As another researcher on the project, Dr. Tan Sui, explained: “By providing better cell-material characterizations, we can shed more light on the way cells interact with each other. This nano-scale analysis could help researchers use nature to inspire better tissue engineered scaffolds, a key pathway to improving screening and treatment. There’s still a lot of work to do before patients benefit, but we’re itching forwards in the right direction.”
Tests to allow a major advance
As reminded in the study’s abstract, the researchers’ “multi-level analysis of structural-mechanical relations in this polyurethane-based pancreatic cancer tissue model opens an opportunity in designing mechanically robust cost-effective tissue models not only for fundamental research but also for treatment screening”.
Pancreatic cancer is particularly aggressive.
As a result, despite the scientific community’s best efforts, survival rates have not drastically improved. For the United-Kingdom, only eight percent of patients have survived for more than five years after being diagnosed. Pancreatic cancer is ranked fifth in the number of cancer-related deaths.
A better understanding of the most dangerous cancers and how they interact with the body may not lead to immediate improvement of patients’ lives, but down the road, it is sure to yield crucial advancements in therapy, screening and prevention of not only this particular form of cancer, but potentially others as well.