The treatment of diseases by inducing, enhancing, or surpressing an immune response is referred to as Immunotherapy. Therapeutic manipulation of immunopathways has led to promising clinical results for the treatment of a number of diseases. The first therapeutic antibodies directed against the checkpoint receptor PD-1 have been already brought to the market (Nivolumab, Pembrolizumab) by Bristol Myers Squibb and Merck/MSD respectively, and approved for the treatment of diverse cancer types.
Today, I would like to review a versatile tool to conduct cell based inhibitor screenings on PD-1 / PD-L1/PD-L2 binding.
T-cell activation and inactivation require the coordination of various co-inhibitory and co-stimulatory signals which are modulated by most immunotherapeutic approaches. The binding of Programmed Cell Death Protein 1 (PD-1), a receptor expressed on activated T-cells, to its ligands, PD-L1 and PD-L2, negatively regulates immune responses. The PD-1 ligands are found on most cancers, and PD-1:PD-L1/2 interaction inhibits T cell activity and allows cancer cells to escape immune surveillance. The PD-1:PD-L1/2 pathway is also involved in regulating autoimmune responses, making these proteins promising therapeutic targets for a number of cancers, as well as multiple sclerosis, arthritis, lupus, and type I diabetes. Further research projects in this field are rapidly evolving as scientists seek to identify the next generation of therapies, especially developing therapies combining different therapeutic approaches.
In a series of posts in 2015 (summarized here in 9 pathway-specific screening assays in Immunotherapy), I looked at biochemical binding assays launched by BPS Biosciences to measure the inhibitory effects of potential drugs on e.g. PD-1/PD-L1/PD-L2 binding and the binding of other checkpoint proteins to their respective ligands, such as CTLA-4/B7-1/B-7.2 and CD28/B7-1/B-7.2 (see Figure 1).

How to run cell based screening assays on PD-1 / PD-L1/PD-L2 binding
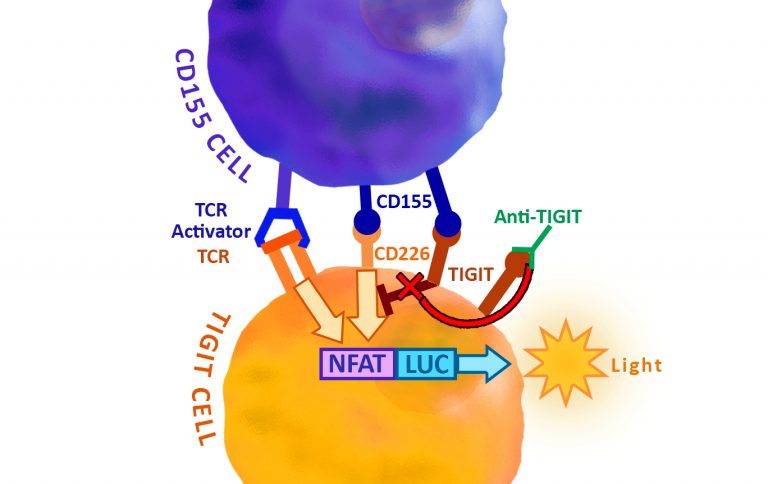
BPS Biosciences have now developed a Jurkat T cell expressing firefly luciferase gene under the control of NFAT (Nuclear Factor of Activated T-cells) response elements with constitutive expression of human PD-1. The principle of the assay which can be done with this cell line is shown in Figure 2.
The functionality of the cell line was validated using a PD-1:PD-L1 (or PD-L2) cell-based assay. In this assay, PD-1/NFAT Reporter/Jurkat T cells are used as effector cells; HEK293 cells over-expressing PD-L1 (or PD-L2) and an engineered T cell receptor (TCR) activator are used as target cells. When these two cells are co-cultivated, TCR complexes on effector cells are activated by TCR activator on target cells, resulting in expression of the NFAT luciferase reporter. However, PD1 and PD-L1 (or PD-L2) ligation prevents TCR activation and suppresses the NFAT-responsive luciferase activity. This inhibition can be specifically reversed by anti-PD1 or anti-PD-L1 antibodies. PD1/PD-L1 neutralizing antibodies block PD1:PD-L1 interaction and promote T cell activation, resulting in reactivation of the NFAT responsive luciferase reporter.

In Figure 3A, the characterization of biological activity of anti-PD-1 neutralizing antibody in PD-1/PD-L1 cell-based assay using the PD-1/NFAT Reporter-Jurkat cells is shown. In this case HEK293 cells were transiently transfected with human PD-L1 and an engineered T cell receptor (TCR) activator. The next day, PD-1/NFAT Reporter-Jurkat cells (or control NFAT Reporter –Jurkat cells) were pre-incubated with anti-PD-1 neutralizing antibody for 30 minutes prior to co-culture with transfected HEK293 cells. After ~16 hours of stimulation, ONE-StepTM Luciferase reagent was added to the cells to measure NFAT activity. Figure 3B shows control results with NFAT Reporter-Jurkat cells not expressing PD-1. Very similar results could be obtained using HEK293 cells transiently transfected with human PD-L2 and an engineered T cell receptor (TCR) activator (data not shown, but shown in the manual of the PD-1 / NFAT Reporter – Jurkat Cell Line)
What can you use these cell lines for?
- Screening for activators or inhibitors of PD-1 signaling in a cellular context
- Characterization of the biological activity of PD-1 and its interactions with ligands