Personalized treatment for cancer reached a new milestone with the FDA approval of CAR T-cells for treatment of leukemia and lymphoma. There is now rapid growth in research on the therapeutic uses of CAR T-cells.
To help researchers develop efficient CAR T-cells, we aim to provide many innovative solutions, such as recombinant cell line expressing cancer antigens, or recombinant versions of these antigens. Developed by BPS Bioscience, these products will help scientists to more precisely monitor the effectiveness of the engineered CAR T to detect and bind to a specific cancer antigen.
CAR T is a cross of immunotherapy, gene therapy, and cellular therapy. As you know, many successful immunotherapies are based on checkpoint inhibitors that block the mechanisms that tumour cells use to hide from T cells. CAR T immunotherapies go one step further by engineering T cells to enhance immune response against a specific tumor antigen.
CAR T is a promising approach for cancer and other diseases, especially severe cancers that do not respond well to other treatments. Huge remission rates of up to 94% are observed in clinical trials of CAR T, and two therapies are already FDA approved (Novartis, Gilead). There is even more promise from over 240 CAR T clinical trials in progress.

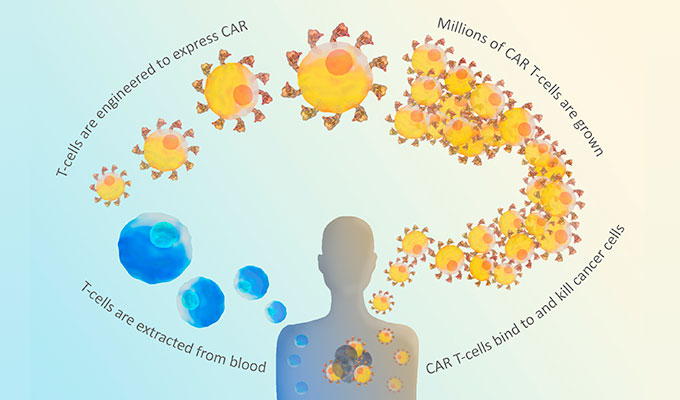
CAR stands for Chimeric Antigen Receptor. CARs are synthetic receptors built to recognize a particular cancer antigen. The CAR is cloned and expressed in T cells removed from the patient (Fig. 1). These cells are tested to make sure they are expressing the CAR, and the resulting CAR T-cells are infused back into the patient to directly target antigen-expressing cancer cells. Both of the two FDA-approved CAR T therapies target a B cell antigen called CD19, as a marker for B cell leukemias and lymphomas.

CARs are designed to include the single-chain variable fragment (scFV) of a monoclonal antibody (Fig.2). They also contain intracellular signaling domains, such as the T-cell receptor CD3ζ chain, that mediates T cell activation, cytokine release, and cytotoxicity when bound to a target cell. Since the CAR already includes the CD3 chain, the T cell doesn’t require the MHC/TCR step for activation. The CAR not only directs the T cell to the cancer antigen, it also activates the T cell to kill it.
 Unfortunately, there can be serious side effects from CAR T including death. The worst cases result from uncontrolled cytokine release in what is known as cytokine release syndrome. Much of the research on second generation CAR T is toward minimizing these potential side effects. Additional regulation comes by adding a second signaling domain such as CD28, associated with the clonal expansion of T cells, or 4-1BB (CD137), associated with longer duration in circulation (Fig. 3). Third generation CAR improvements include signaling domains such as CD27, CD28, ICOS, and OX40, which improve targeting of the CAR T cell. Not surprisingly, many of these extra signaling domains are derived from important checkpoint inhibitor pathways. Some of the clinical trials are investigating combination therapies by including CAR T with chemotherapy or with checkpoint inhibitors (immunotherapy). Also, since CAR T therapy is not MHC-dependent, other researchers are developing “off the shelf” CAR T from healthy donors that can be used in any patient whose cancer is expressing that particular cancer antigen recognized by the CAR T.
Unfortunately, there can be serious side effects from CAR T including death. The worst cases result from uncontrolled cytokine release in what is known as cytokine release syndrome. Much of the research on second generation CAR T is toward minimizing these potential side effects. Additional regulation comes by adding a second signaling domain such as CD28, associated with the clonal expansion of T cells, or 4-1BB (CD137), associated with longer duration in circulation (Fig. 3). Third generation CAR improvements include signaling domains such as CD27, CD28, ICOS, and OX40, which improve targeting of the CAR T cell. Not surprisingly, many of these extra signaling domains are derived from important checkpoint inhibitor pathways. Some of the clinical trials are investigating combination therapies by including CAR T with chemotherapy or with checkpoint inhibitors (immunotherapy). Also, since CAR T therapy is not MHC-dependent, other researchers are developing “off the shelf” CAR T from healthy donors that can be used in any patient whose cancer is expressing that particular cancer antigen recognized by the CAR T.
Evaluating CAR expression is essential for the production of CAR T-cells. BPS has created cell lines expressing important cancer antigens, enabling researchers to demonstrate that their CAR T-cells are specific for particular targets. Beyond CD19, these cell lines express BCMA, CD47, ERBB2, CD20, PD-L1, CD22, ROR1, CD37, SLAMF7, and CD38.

Cancer cells express many different kinds of antigens, including tumor-specific antigens (TSA), unique to tumor cells, and tumor-associated antigens (TAA) which are more general (Fig 4). TAAs are normally expressed only in certain cell types or during certain states of development. Many cancer cells also express mutated versions of normal proteins associated with growth, proliferation, and differentiation, which allows them to rapidly multiply and metastasize.
Importantly, the expression level of these antigens can change significantly over time, depending on the type of the tumor and the stage of the cancer. Expression levels also correlate with tumor progression and prognosis, and the level of certain cancer antigens can be used to monitor the success of therapeutic treatments.

Flow cytometry using PE-conjugated anti-human BCMA detects BCMA surface expression of BCMA-CHO recombinant cell lines with different expression levels: #14979500-H, high expresser: green; #14979500-M, medium expresser: purple; #14979500-L, low expresser: brown; wildtype CHO negative control: red.
BPS has developed stable, recombinant cell lines expressing cancer antigens at different expression levels: high, medium, or low expression (Fig. 5). These cell lines can be used to monitor the effectiveness of different CAR T at detecting the target antigen expressed at different levels, which may relate to the time dependence of antigen expression. The image shows each of these cell lines detected by a PE-labeled antibody against the target antigen BCMA, vs. the control cell line.
BPS also offers labeled versions of many of the antigens recognized by CAR T-cells, such as BCMA, CD19, CD22, CD123, CD38, and ROR1 among many others. These labeled proteins can bind to CAR T-cells and be detected by flow cytometry using PE-streptavidin, in order to verify that the CAR T cell is recognizing and binding the specific cancer antigen.
Engineering T cells to boost the immune system has potential benefits beyond cancer treatment, also including treatments for chronic inflammatory diseases such as hepatitis, HIV/AIDS, lupus, and arthritis. Companies are also investigating CAR T cell therapy in organ transplantation to eliminate the need for lifelong immuno-suppressants. The future is bright for CAR T research, and in collaboration with BPS Bioscience, tebu-bio will continue to distribute unique cell lines and other tools to help researchers create, evaluate, and enhance CAR T-cells for the improvement of human health.
Check all our innovative CAR T-cell solutions directly on our web site or contact your local office for more information.
Interested by CAR T cell therapy? Check the following topics which will also be of interest to you.