In vitro transcription has been a common protocol in RNA biology laboratories wishing to work directly with mRNA molecules to study phenomena such as mRNA translation. Commercially available kits have greatly facilitated the capping and polyadenylation and in vitro transcription of RNAs, but now there is another option: ordering chemically synthesized mRNAs.
In vitro transcription kits such as the T7-FlashScribe™ Transcription Kit allow maximum RNA yields in 30 minutes. Subsequent processing of primary transcripts involves addition of the 5′ cap and 3′ poly(A) tail.


Some of the most popular kits for mRNA processing include the ScriptCap™ m7G Capping System for capping and the A-Plus™ Poly(A) Polymerase Tailing Kit for tailing. Researchers wishing to optimize protein expression use chemically-modified mRNAs, such as those carrying an anti-reverse cap analog ARCA at the 5′ end. Again, kits such as the MessageMAX™ T7 ARCA-Capped Message Transcription Kit have made in vitro transcription of ARCA capped mRNAs routine in laboratories. When a standard m7 GTP cap is added to mRNAs in vitro, only about 1/2 of the cap is added in the correct orientation. The ARCA cap is modified with a methyl group to prevent capping in the “incorrect” orientation, thus resulting in a higher percentage of efficiently translated mRNA.
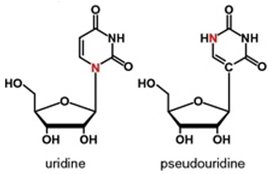
Depending on the application, researchers are seeing advantages of using other chemical modifications as well. Pseudouridine-5′-Triphosphate, a naturally occurring base, is used to decrease nuclease activity and TLR activation, and modified cytidine base (5-Methylcytidine-5′-Triphosphate) is used for similar reasons. Warren et al. 2010 (doi: 10.1016/j.stem.2010.08.012), for example, found that mRNAs carrying an ARCA cap and Pseudo-uridine and methyl-cytidine substitutions are very efficient for reprogramming of many human cell types and fail to activate the toll-like receptor innate immune pathways.
Similarly, the Immune Stimulation Transcription Nucleotide Set is available for those who want to purchase ARCA cap, Pseudouridine-5′-Triphosphate, and 5-Methylcytidine-5′-Triphosphate. Another modification gaining popularity is the modified uridine (2-Thiouridine-5′-Triphosphate), however this popularity may be a result only of intellectual property limiting the commercial use of Pseudo-uridine.
Commercially-available capped and polyadenylated mRNAs modified with pseudouridine and 5-methylcytidine include those encoding:
Gene |
Pseudouridine |
5-methylcytidine |
Length (nucleotides) |
| EGFP mRNA (5meC, Ψ) | + | + | 996 |
| Oct4 mRNA (5meC, Ψ) | + | + | 1,359 |
| Klf4 mRNA (5meC, Ψ) | + | + | 1,688 |
| SOX2 mRNA (5meC, Ψ) | + | + | 1,230 |
| c-Myc mRNA (5meC, Ψ) | + | + | 1,596 |
| Lin28 mRNA (5meC, Ψ) | + | + | 906 |
| FLuc mRNA (5meC, Ψ) | + | + | 1‚929 |
| NLS-Cre mRNA (5meC, Ψ) | + | + | 1‚350 |
| β-gal mRNA (5meC, Ψ) | + | + | 3,336 |
| Factor IX mRNA (5meC, Ψ) | + | + | 1,662 |
| hAAT mRNA (5meC, Ψ) | + | + | 1,530 |
| mCherry mRNA (5meC, Ψ) | + | + | 996 |
| Eira CFP mRNA (5meC, Ψ) | + | + | 978 |
| Blaze YFP mRNA (5meC, Ψ) | + | + | 987 |
| Cas9 Nickase mRNA (5meC, Ψ) | + | + | 4,341 |
| EPO mRNA (5meC, Ψ) | + | + | 858 |
| CD8 mRNA (5meC, Ψ) | + | + | coming soon |
| NGFR mRNA (5meC, Ψ) | + | + | coming soon |
| Guassia Luciferase mRNA (5meC, Ψ) | + | + | 834 |
| Renilla Luciferase mRNA (5meC, Ψ) | + | + | 1,212 |
| Cas9 mRNA (5meC, Ψ) | + | + | 4,509 |
| EGFP mRNA (5meC) | + | 996 | |
| FLuc mRNA (5meC) | + | 1‚929 | |
| OVA mRNA (5meC, Ψ) | + | + | 1,437 |
| Cas9 mRNA (Ψ) | + | 4,509 | |
| EGFP mRNA | 996 | ||
| FLuc mRNA | 1‚929 | ||
| β-gal mRNA | 3,336 | ||
| OVA mRNA | 1,437 | ||
| Cyanine 5 FLuc mRNA (5meC, Ψ) | + | + | 1‚929 |
| Cyanine 5 EGFP mRNA (5meC, Ψ) | + | + | 996 |
Clearly, many of these purified mRNAs encoding fluorescent proteins, luciferase, or other reporter proteins are intended as controls for users setting up assays on which disease-relevant mRNAs will be tested. Once the assays are established, users now have the choice to produce their mRNAs of interest themselves using the in vitro transcription, capping, and polyadenylation kits described above or to order high quality mRNAs produced by expert chemists. Chemically synthesized mRNAs can be synthesized to contain nearly any sequence and chemical modification desired and gram quantity yields are possible. For particularly long mRNAs (up to multiple kilobases), in vitro transcription steps may still be required, but experts at TriLink Biotechnologies are able to design custom strategies to optimize yield even for the most complicated custom mRNA production requirements.
European scientists interested in learning more about out-sourcing mRNA production are encouraged to contact the local TriLink Biotechnologies distributor, tebu-bio.