Advances in mRNA Vaccines for Infectious Diseases
It is now admitted that mRNA vaccines provide a safe and long-lasting immune response in humans. Since capping of the mRNA is involved in the protein production into the cells, the capping technologies are of great interest for the development of prophylactic and therapeutic vaccines. In this post, I’ll focus on the CleanCap reagents (Trilink BioTechnologies), and notably the CleanCap AU for the self-amplifying mRNA, as well as their benefits, especially for vaccine approaches against pathogens (including the SARS-CoV-2).
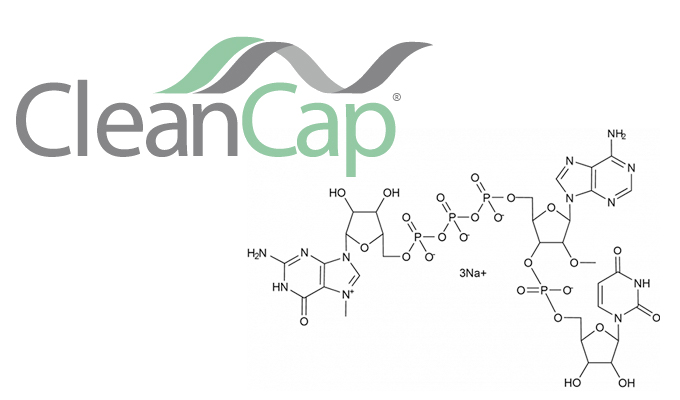
CleanCap – a new standard for superior translation
CleanCap reagents are simply used in the in vitro transcription as nucleotide analogs. Unlike enzymatic capping, the co-translational method is done in one simple reaction.
mRNA produced with CleanCap reagents provide superior in vivo activity than the classic co-transcriptional capping methods (such as with ARCA analogue) as shown below.

Indeed, the ARCA leads to CAP0 structure. To obtain, the natural CAP1 structure with a co-transcriptional method, the unique solution is CleanCap.
CleanCap AG mRNAs have been shown with less toxicity and higher protein expression, especially combined with 5moU modification as illustrated below.


CleanCap mRNAs have also higher stability.

CleanCap reagents allow Life Scientists to produce the most active, longest lasting and least toxic mRNA for in vitro and in vivo applications.
CleanCap AU for the capping of self-amplifying mRNA
The CleanCap AG reagent was the 1st released in 2017. It became rapidly a real standard for mRNA capping for academics and industrial customers with a capping efficiency (CAP1) of 96% in one reaction. Such capping requires an AG initiator. There is more detailed information in my post on CleanCap AG. Already, many therapeutic vaccine developments benefit from this CleanCap AG reagent.
Since the most popular initiator of T7 promotor is GG, Trilink also produces a CleanCap GG. Unlike ARCA, it allows CAP1 structure. Nevertheless, the capping efficiency is the same than ARCA.
Today, the CleanCap AU reagent with AU initiator is now available.

The AU initiator is found in viral RNA able to be amplified into the host cells thank to a RNA dependent RNA-polymerase. it is a kind of boost of the mRNA pool leading to high viral protein production.
If we replace the ORF sequence of a viral structure protein by another ORF coding for a protein of interest, the resulting self-amplifying mRNA would produce the protein of interest at high level, mimicking the viral infection boost. It is clearly of high interest for prophylactic vaccines and possibly also to therapeutic vaccines, against cancers for example.
We offer the CleanCap reagents as well as custom mRNA production services. You don’t need to be expert – we will provide assistance.