Extracellular vesicles in general, or exosomes in particular, are becoming a hot topic for research, especially in cancer. They seem to have different roles in tumour progression or can be used as targets to develop new therapies. In our series of posts on exosomes, we will focus today on their role in signal transduction.

Knowledge of the content of exosomes, and their role to in cell-to-cell communication by mediation of signal transduction, is of interest on two sides.
On one hand, exosome content can be a source of biomarkers. Exosomes are present in a variety of fluids, such as blood, urine, saliva and cerebrospinal fluid, and they contain proteins, lipis, mRNA and miRNA. The study of disease-specific exosome content allows to find novel biomarkers to study the onset and progression of several diseases.
On the other hand, exosomes can be used as gene delivery vehicles, and their immunomodulatory and regenerative properties are encouraging their application for therapeutic purposes (1). However, much research is needed until we reach that stage. And your laboratory can be part of advancements related in this area.
Exosomes can interact with other cells by several means. One is interaction with membrane receptors on target cells. Another hypothesised mechanism is based on soluble ligands produced by proteolytic cleavage of exosomal membrane proteins. And the third mechanism is internalisation (2).
The discussion lies not so much in whether exosomes have an effect on cell signaling, as it is quite clear that they mediate signal transduction in both autocrine and paracrine fashion by the transfer of proteins and RNA (mRNA or miRNA). Rather, discussion lies in whether this effect is pathogenic or protective (3). Also, exosomes seem to be involved not only in pathogenic states and/or disease progression, but also on homeostasis.
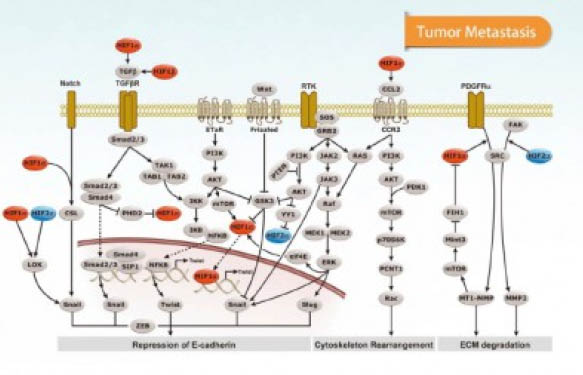
As for signal transduction, it seems that several pathways have a role in exosome-mediated cell-to-cell communication, including EGFR and other RTK-mediated signaling, PI3K, Wnt/Notch/β-catenin, Ser/Thr kinase receptors (e.g. TGF-β/activin/BMP), GPCRs, toll-like, NFkB, p53, etc. (4).
Let’s take RTK signaling. Exosomes can either play an inhibitory effect in RTK signaling by sequestering activated receptors, preventing their access to cytoplasmic signaling components. But also, exosomes can play a positive role in switching on a signaling pathway through sequestration of an inhibitor (e.g. in Wnt signaling, where GSK3 is hidden inside internal vesicles). Cross-talk between exosomes and endosomal compartments, as well as release of new exosomes, is a key process in cell-to-cell communication, and still much needs to be elucidated. Also mainly because each cell type and each physiological status seem to have their own regulatory mechanisms.
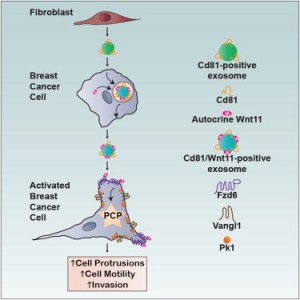
For example, in breast cancer, cell migration seems to be mediated by exosomes though the Wnt-PCP (planar cell polarity) pathway (5). Breast cancer cell (BCC) protrusions are stimulated by exosomes, and in mouse models of breast cancer, metastasis is dependent on PCP signaling in BCC and the exosome component, Cd81 in fibroblasts. Trafficking in BCCs promotes tethering of autocrine Wnt11 to fibroblast-derived exosomes. Therefore, fibroblast exosomes mobilise autocrine Wnt-PCP signaling to drive BCC invasive behaviour.
So, if you think that exosomes play a role in your experimental model, there are several approaches you can take, depending on the existing knowledge you have about the relevant signaling pathways in your homeostatic or pathologic model. If you are working on translational medicine, you may decide to study only those pathways found in animal models to the human samples, or you may decide to explore a bit further. You may decide to do WBs, IHCs or P-ELISAs only on those kinases you already know, or explore new pathways by performing profiling antibody arrays that allow to study more than one thousand signaling biomarkers.
Let me insist, much research is still needed in exosomes until we reach the stage to use them as a source of biomarkers or new therapies, and you can play a role in these new findings!
Ready to explore?
References