The Alvetex®Scaffold is a novel substrate that enables a solution for simple and routine 3D culture. It is composed of a highly porous polystyrene scaffold that has been engineered into a 200 micron thick membrane to enable entry of cells and efficient exchange of gases and solutes. Cells enter the fabric of the scaffold, retain their natural 3D structure, and form close 3D interactions with adjacent cells. Unlike conventional 2D culture, cells in Alvetex®Scaffold do not grow as monolayers and do not undergo the flattened shape transition that can result in aberrant changes to gene and protein expression and consequently cellular function.
Mesenchymal stem cells (MSCs) are adherent multipotent cells derived from tissue such as bone marrow and which possess the ability to differentiate in vitro into a number of tissue types including bone, cartilage and muscle [1].
In this post, we demonstrate that MSCs extracted from the bone marrow of adult rats can be successfully cultured in 3D in Alvetex®Scaffold and induced to differentiate into osteogenic and adipogenic derivatives more efficiently than their 2D counterparts. We also report bone formation and the production of extracellular matrix by MG63 cells which represent an established cell line derived from a human osteosarcoma. The data generated here is supported by peer-reviewed literature [2,3] and clearly shows that Alvetex®Scaffold promotes enhanced in vitro differentiation.
3D Culture of Mesenchymal Stem Cells in Alvetex®Scaffold
Populations of rat primary MSCs grown on Alvetex®Scaffold showed a consistent and increasing growth pattern over the 14 day test period (Figure 1A). Cell viability was assessed by MTT assay. Absorbance values increased in a linear fashion and did not plateau during this period suggesting further growth potential. Staining the 3D culture with Neutral Red showed the gross distribution and density of MSCs in the scaffold (Figure 1B). After 14 days cells were located over the majority of the Alvetex®Scaffold disc, although there remained some space for further expansion of the cell population around the periphery. Histological analysis revealed the microscopic distribution of 3D cells inside the scaffold (Figure 1C). MSCs show a relatively homogeneous distribution but do not pack closely together at this stage and therefore cell-cell contact inhibition of growth is avoided. This enables the continued 3D expansion of the cell population over the 14 day culture period.

Osteogenic Differentiation of MSCs in Alvetex®Scaffold
Cultured rat MSCs grown in 3D on Alvetex®Scaffold were induced to differentiate into bone-forming cells following a standard procedure. Analysis of the expression of markers associated with osteogenesis was performed and demonstrated the differentiation of bone-producing cells (Figure 2). Compared to cells grown on 2D plastic, cells grown on Alvetex®Scaffold deposited more calcium and maintained stable collagen production which is indicative of osteogenic rather than chondrogenic differentiation. Furthermore, the culture of MSCs on Alvetex®Scaffold without any osteogenic stimulus was enough to induce the deposition of calcium (data not shown). The production of alkaline phosphatase in response to incubation in osteogenic medium was increased in cells growing on Alvetex®Scaffold compared with cells cultured on 2D substrates. Alkaline phosphatase is involved in the regulation of phosphate availability for hydroxyapatite production [4]. It is likely therefore that the higher levels of alkaline phosphatase in 3D culture contributed to the greater amount of calcium deposited in Alvetex®Scaffold cultures compared to their 2D counterparts. Osteocalcin was also greatly increased in cells differentiated on Alvetex®Scaffold, which is associated with activation of insulin signalling and bone matrix remodelling via resorption in vivo [5].

Histological analysis of rat MSCs differentiating into bone during 3D culture on Alvetex®Scaffold was performed using standard tissue fixation, processing and staining procedures. Wax embedded sections were processed with different stains to show deposition of various materials associated with osteogenesis. Von Kossa staining showed the presence of calcium deposits whilst histological counterstaining demonstrated the presence of collagen (Figure 3). Histological detection of markers such as calcium and collagen supports the quantitative data presented in Figure 2, indicating bone formation in 3D culture using Alvetex®Scaffold technology.

Differentiation of Human Osteosarcoma Cells on Alvetex®Scaffold
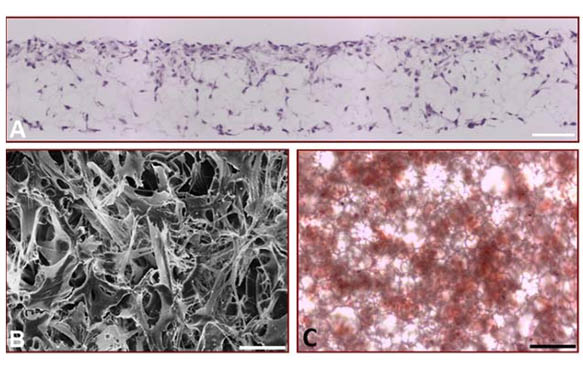
Alvetex®Scaffold is a highly versatile technology able to support bone formation by different cell types including those derived from healthy or pathological bone tissues. For example, cell lines established from osteosarcomas are often used to study features of bone cell differentiation, function and disease. Here we also demonstrate the application of Alvetex®Scaffold technology to support 3D culture of the osteosarcoma cell line, MG63 (Figure 4). These data demonstrate the ability of MG63 cells to grow and proliferate throughout the scaffold. Histological data (Figure 4A) shows that the pattern of MG63 cell growth closely resembles that seen with the MSCs (Figure 1C). Scanning electron microscopy reveals more clearly the complexity of MG63 cell morphology in 3D culture and the interactions between cells. Assessment of calcium deposition by Alizarin Red S staining was evident throughout the culture and easily detectable by standard light microscopy. Compared to MG63 cells grown on conventional 2D plastic, those grown on Alvetex®Scaffold exhibited greater alkaline phosphatase activity and produced more osteocalcin.

(A) Micrograph of H&E stained, wax-embedded sections from MG63 cells cultured in Alvetex®Scaffold for 7 days; (B) Scanning electron micrograph of MG63 cells cultured in Alvetex®Scaffold as viewed from above; (C) Light micrograph of Alizarin Red S staining showing the gross distribution of calcium deposition by MG63 cells cultured in Alvetex®Scaffold for 21 days. This image was taken from directly above the scaffold disc; (D) The expression of alkaline phosphatase activity in differentiating MG63 cells grown on Alvetex®Scaffold for up to 21 days was assayed by dephosphorylation of p-nitrophenyl phosphate substrate, normalised to average total protein content. Data represent average nmol of alkaline phosphatase per mg of total protein per hour, n=3, ±SEM; (E) The concentration of osteocalcin released into the medium was determined by ELISA and normalised to average total protein content. Data represent average ng of osteocalcin per mg of total protein, n=3, ±SEM. Total protein levels were determined by Bradford assays. Scale bars: 100 µm (A); 20 µm (B); 50 µm (C).
Adipogenic Differentiation of MSCs in Alvetex®Scaffold
We have demonstrated the application of Alvetex®Scaffold to support the differentiation of bone. The application of Alvetex®Scaffold to support adipogenesis and the formation of fat-producing cells was also investigated (Figure 5). Rat MSCs were grown on Alvetex®Scaffold and induced to differentiate toward the adipogenic lineage using a standard method. Samples of the differentiated cells were then successfully retrieved from the scaffold and subsequently cytospun onto microscope slides and stained for analysis. The data show a greater number of lipid droplets present in individual cells grown in 3D culture using Alvetex®Scaffold compared to equivalent cells grown in conventional 2D culture. This observation was confirmed and quantified using an alternative approach that measured the absorbance of Oil Red O staining in samples extracted directly from the two culture methods. The data were normalised to take into account any differences in cell numbers. Overall, the results demonstrate that MSCs differentiating toward the adipogenic lineage produce greater quantities of lipid when grown in 3D culture compared to their counterparts maintained in conventional 2D plastic-ware. Such differences were detectable after 7 days after induction of differentiation and were maintained for up to 21 days. This outcome suggests that 3D culture of rat MSCs favours improved cell growth and differentiation.

Conclusions
This application note describes the use of Alvetex®Scaffold to support the differentiation of mesenchymal cells towards osteogenic and adipogenic phenotypes by providing a 3D cell culture environment. Both MSCs and MG63 cells readily grow and proliferate in the scaffold, establishing genuine 3D cultures. The data presented demonstrates that 3D culture using Alvetex®Scaffold radically improves the ability of these cells to form mesenchymal tissues such as bone and fat. Superior cell morphology is achieved in 3D culture that is likely to contribute to the enhanced cell function as seen by increased levels of osteogenic and adipogenic markers. Cells from primary sources and established cell lines have been used which underlines the versatility of Alvetex®Scaffold technology for alternative applications. A range of different analytical methods has been used to test and evaluate the status of cell growth and differentiation, highlighting the compatibility of Alvetex®Scaffoldwith standard molecular and cellular techniques. Overall, Alvetex®Scaffold provides a simple and robust approach for routine 3D cell culture. It is a convenient tool for promoting both osteogenic and adipogenic differentiation by mesenchymal cells. The highly porous structure of Alvetex®Scaffold and range of alternative formats provide new opportunities for maintaining cells in 3D culture and creating more physiologically relevant in vitro models and the formation of tissue-like structures.
References
- Pittenger, M.F., A.M. Mackay, S.C. Beck, et al., Multilineage potential of adult human mesenchymal stem cells. Science, 1999. 284(5411): p. 143-7.
- Neofytou, E.A., E. Chang, B. Patlola, et al., Adipose tissue-derived stem cells display a proangiogenic phenotype on 3D scaffolds. Journal Biomedical Materials Research Part A, 2011. 98(3): p. 383-93.
- Bokhari, M., R.J. Carnachan, S.A. Przyborski and N.R. Cameron, Emulsion-templated porous polymers as scaffolds for three dimensional cell culture: effect of synthesis parameters on scaffold formation and homogeneity. J. Mater. Chem., 2007. 17: p. 4088-94.
- Orimo, H., The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon. Med. Sch., 2010. 77(1): p. 1-12.
- Ducy, P., The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia, 2011. 54: p.1291-7.
- Croft, A.P. and S.A. Przyborski, Formation of neurons by non-neural adult stem cells: potential mechanism implicates an artefact of growth in culture. Stem Cells, 2006. 24(8): p. 1841-51.