mRNA vaccines are developing very quickly with dozens of ongoing clinical trials against cancers or infectious diseases (e.g. HIV or more recently SARS-Cov-2). Recombinant monoclonal antibody (mAB) based treatments have proven their efficacy. Nevertheless, researchers are now considering using messenger RNA (mRNA) technologies for the development of prophylactic and therapeutic vaccines.
Pre-clinical and clinical trials have shown that mRNA vaccines provide a safe and long-lasting immune response. These vaccines are now clearly considered an efficient approach to developing prophylactic vaccines but also therapeutic ones to fight against infectious agents (e.g. COVID-19 with Moderna’s mRNA-12173 and BioNTech’s BNT162 vaccine candidates or against cancer cells by expressing tumor-associated antigens. mRNA vaccines can also encode the light and heavy chains of a broadly neutralizing antibody against the virus.
These New Biological Entities (NBE) have several advantages over conventional drugs:
- Prompt and flexible design
- Cost and speed production
- Controlled administration root…
They provide thus a realistic and practical approach to personalized medicine. A major step forward was done by BioNTech, in 2016, with the first successful mRNA vaccine against skin cancers (Nature, June 2016, doi: 10.1038/nature18300). The authors delivered mRNA encoding for specific cancer cells proteins to antigen-presenting cells (APCs) in lymphoid organs to trigger a specific immune response of the patient’s body against cancer cells. A year after, the authors went further with the concept of individualized mutanome vaccines, which are RNA-based treatments to drive immunity against a spectrum of cancer mutations (Nature, July 2017, doi: 10.1038/nature23003).
The use of mRNAs in drug discovery is less restricted than in the past when various hurdles were limiting their use (e.g. instability inside the cells, high innate immunogenicity with the Cytokine Syndrome Release (a.k.a. “Cytokine Storm”), in vivo delivery, …). From a practical point of view, it was also not easy to produce proper amounts of mRNA. A certain level of expertise was also required, explaining why DNA-based and protein-based therapeutics were preferred approaches.
Fortunately, technological breakthroughs and progress in chemistry have modified the landscape.
Recent signs of progress for efficient RNA-based drug discovery
Capping protection and stability
Protection of the mRNA depends notably on the capping structure in 5′. The ARCA method is classic and popular. It provides a CAP0 structure which is a “pretty good” RNA protection method. Nevertheless, the capping efficiency is only around 60-70%.
TriLink has developed modified mRNA with another type of reagents called CleanCap. Capping efficiency is far better (>90%). The structure is CAP1. It corresponds to the natural structure we can find in the cells. The translation is also improved providing more protein and so more function.

Major reduction of immunogenicity
The standard to obtain a high reduction of the immunogenicity of the mRNA is to perform HPLC purification. Unfortunately, it requires quite a lot of volume and it is costly.
Several chemical modifications of nucleosides (such as pseudouridine (Ψ), 5-methylcytidine (5 mC)) can avoid mRNA recognition by the sensors of the innate immune sensors reducing thus excessive pro-inflammatory cytokine releases. In parallel, CAP-1 structure and optimized codons improve translation efficiency.
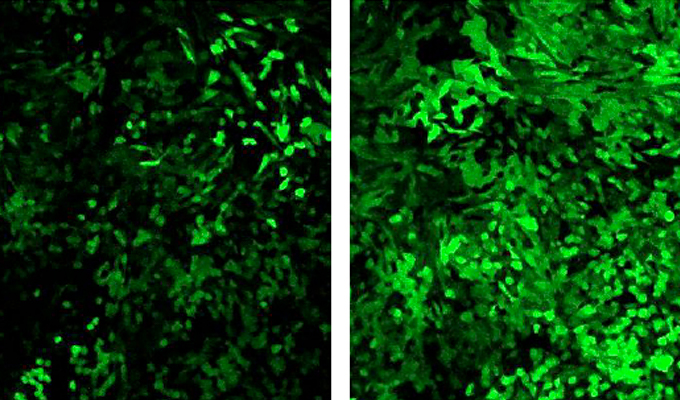
TriLink offers a license-free 5moU modification that has been shown to be as efficient as HPLC purification. And actually, adding HPLC purification to the 5moU modified mRNA further reduces the immune response of the cells. Thus, the 5moU modified mRNA is better accepted by the cells, not promptly degraded, and provides more protein as illustrated below with eGFP mRNA.

Innate immune responses monitoring during mRNA vaccine development
To monitor the innate immune response from the blood (plasma, serum) or cell culture supernatant sample, a high sensitivity multiplex-ELISA is an ideal approach.
To ensure more sensitive and reproducible analysis, I would recommend the Quansys technology for the simultaneous quantification of 16 inflammatory cytokines in one sample: Human Cytokine Release (16 plex).

If you are interested in the monitoring of inflammatory cytokines and IFNS, you might have a look at the following post “How to monitor inflammatory cytokines in anti-COVID drug discovery“. Interestingly, all the profiling, multiplex quantification arrays, or ELISAs are available as ready-to-use assay kits or laboratory services.
Drug delivery optimization
Delivery of the mRNA remains the main challenge. Today, solutions exist from naked RNA to mRNA complexed with cationic lipids, cholesterol, and PEG-lipids. We also find lipid-based nanoparticles including the use of 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE). Several biotech companies have developed their specialties and expertise for that particular drug delivery. And as a matter of fact, each project needs a dedicated answer.
Thus, the best way to help research in this field is certainly to provide ready-to-use control mRNA such as eGFP coding mRNA or Luciferase coding mRNA.
Expert of mRNA production
We can now provide codon optimization, based on U-depletion, to provide the best-capped mRNA. Taking into account the U-depletion, the CleanCap CAP1 structure, the UTRs and polyA tail optimization and even the 5moU modification, is what makes control mRNA the most reliable tools for delivery studies. Remarkably, it is far more reproducible and time-saving than home-made batches with ARCA or enzymatic methods. So you can focus your activities on the development of the nanoparticles.
Since mRNA production is not an easy and straight-forward task, our labs offer custom productions. Custom mRNA benefits from the same optimizations as the CleanCap control mRNA, for the continuation of the drug delivery project to the coded protein of interest. These services provide an efficient, simple, and easy source of robust mRNA with high yield, all for your peace of mind. It’s compatible with challenging projects such as those involving long mRNA up to 15k bases.
From the perspective of clinical trials, researchers can keep the same manufacturing team and benefit from their GMP facilities.
Related products:
Assay services for the simultaneous quantification of type I, II, III Human IFNs and related pro-inflammatory cytokines (IFN-alpha, IFN-beta, IFN-gamma, IFN-lambda, IFN-omega, IL-1alpha, IL-6, IP-10, and TNF-alpha)
from only 50 μl required to quantify all 9 analytes in a single assay.
- Polyethylenimine (PEI)
Let me know your comments, concerns, and challenges through the comment form below, I’ll be pleased to assist you!
By the way, for more info on mRNA vaccines, I would suggest the review of:
- Pardi N. et al. Jan 2018, Nature reviews/Drug discovery (doi:10.1038/nrd.2017.243).
- Zhang C. et al. March 2019, Front. Immunol., (doi.org/10.3389/fimmu.2019.00594).
This post was first published on January 25th, 2018 and subsequently edited and updated on April 24th, 2020.