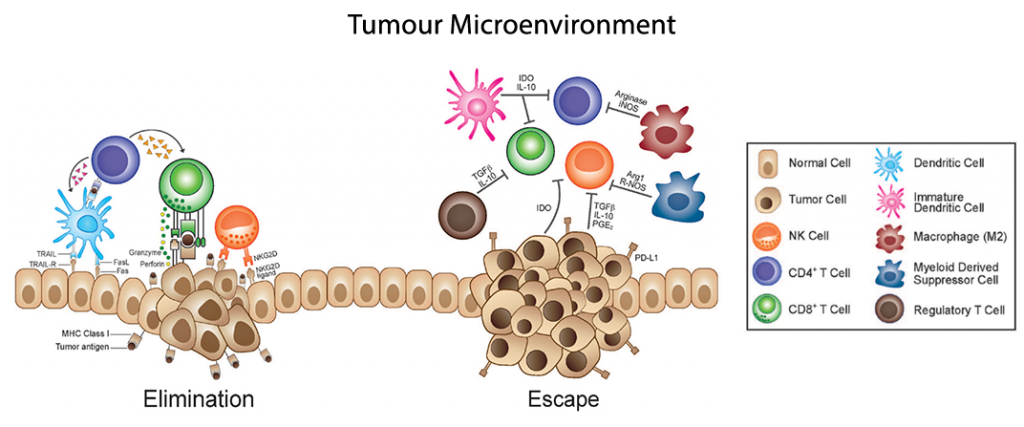
Tumours are composed by a heterogeneous group of cells from diverse organs, ranging from stem cells and endothelial cells, to a wide range of immune cells. The plethora of secretory signals from cancer cells have numerous effects that help promote tumour growth and progression, while also perturbing the immunologic surveillance of developing tumours.
Cancerous cells express their own profile of cytokines and chemokines that facilitate inflammation, cell growth, and recruitment of new blood vessels. It is also recruiting accessory cell populations for their survival and immunologic avoidance. Collectively, these local changes promote the developing tumour microenvironment (TME). As we have seen in previous posts, multiplexed immunoassays remain the best and most complete means to study the proteomic changes within the TME, as they afford the most global view of protein changes from numerous and disparate cell populations.
High-density protein expression profiling is now possible with the latest advancements in multiplex ELISA platforms. They enable the detection of a diversity of novel cytokine interactions in tumour cell populations. As these unique pathways are determined, more traditional biomolecular studies can then define these networks. Multiplex ELISAs and antibody arrays therefore represent powerful tools for the identification of new cancer biomarkers, either from the local TME, or from the cancer cells themselves.
This post is the first one of a series aiming at describing the mechanisms of TME and immunosurveillance and at introducing the reliable immunoassays to analyse cellular crosstalks at the protein level. Thanks to Jarad Wilson, from Raybiotech Inc., for his help on making this series!
Tumour immunosurveillance crosstalks – the main actors to monitor
Tumour immunosurveillance is the identification and elimination of cancer cells by the immune system. This process is predominantly mediated by CD8+ cytotoxic T lymphocytes (CTLs), natural killer cells (NK), neutrophils, and several subtypes of effector CD4+ T cells (CD4s), with accessory roles performed by antibody producing B cells and macrophages (Mφ) amongst others.
Effective immunosurveillance requires the innate immune system’s recognition of the tumour’s presence and the subsequent full activation and maturation of antigen presenting cell (APC) populations, namely the dendritic cell (DC) population. This maturation process increases APC surface expression of MHC-antigen complexes, increases APC endocytic sampling, upregulates cytokines that recruit T cell populations (IL-6, IL-12), and increases surface expression of T cell costimulatory ligands (CD80,CD86, ICOS).
Fully mature DC populations are potent anti-tumour APCs capable of activating all forms of tumour-specific T cell populations. Activated CD8 T cells differentiate to form CTLs which have profound inflammatory and cytolytic functions, while activated effector CD4 T cells secrete cytokines that have immunostimulatory and chemotactic effects.
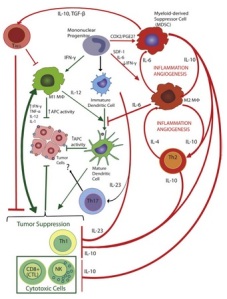
Specifically, effector CD4 T cells develop into a T helper 1 (Th1) population which secretes IL-2 to promote CTL and further CD4 T expansion, TNF-α to inflame the site and recruit other immune cells, and IFN-γ which has anti-tumor and inflammatory functions. IFN-γ also functions to activate and drive Mφ populations into an M1 phenotype, which further produce IL-1α and IL-1β, feeding back to promote Th1 effector CD4+ polarisation and reinforcing the anti-tumour immune programming.
Collectively, these targeted immune responses are capable of shrinking the cancer population, but such a targeted measure can create selective pressures on those tumour cells capable of avoiding this surveillance program. The development of tumorigenesis requires the eventual subversion of immunosurveillance, a multi-step process leading to eventual escape from immunologic recognition and control.
Th1 lymphocytes and M1 Mφ are the primary sources of pro-inflammatory cytokines that promote cancer immunosurveillance and cytotoxicity. Their interactions are mutually reinforcing: Secretion IFN-γ by Th1 cells results in the recruitment and maintenance of M1, while IL-12 produced by M1 macrophages recruits, activates and maintains Th1 cells. Secretion of MIG/CXCL9 and IP-10/CXCL10 also promotes the recruitment of Th1 cells and CTLs and inhibits angiogenesis. IL-1α, IL-1β and IL-6 form an autocrine feedback loop by stimulation of myeloid differentiation primary response gene 88 (MyD88)-mediated activation of NF-κB signaling. TNF-α, also released by the activation of NF-κB signaling, which activates APC functions of DCs and the recruitment and cytotoxic activation of M1 macrophages, effector CD4+ T cells, and CD8+ cells, as well as the recruitment of NK cells.
Th2 lymphocytes, M2 macrophages and MDSCs mutually reinforce the proliferation and phenotypes of one another, as well as maintaining tumor-promoting inflammation and angiogenesis. These cells, along with T Regulatory lymphocytes (TREGs) suppress the activity and proliferation of tumor-suppressing cells, including Th1, M1 and cytotoxic T cells and NK cells.
It should be noted that M1 &M2 Mφ can interconvert, but these phenotypes are stable as the M1 and M2 expression profiles reinforce their own macrophage phenotypes, while suppressing the other. Similarly, Th1 & Th2 lymphocytes, as well as TREG & Th17 lymphocytes tend to self-reinforce their own activation profiles and inhibit the other.
Having a look at this picture, one would think that the immune system controls the growth and dissemination of cancer cells. We know that, unfortunately, this is not always true.
So…what happens when immunosurveillance fails and why does it fail? Stay tuned for our next post!
Looking for validated immunoassays to analyse tumour crosstalks? Please do not hesitate to leave a message below.