Originally identified as an active molecule against the Borrelia species, Borrelidin has since been described as a selective inhibitor of threonyl tRNA synthetase (ThrRS). More recent research has found that Borrelidin (also called Treponemycin, Antibiotic U 78548 or C2989) induces the collapse of newly formed capillary tubules, exhibits anti-bacterial, -malarial, -insecticidal activities, and displays a potent anti-VEGF induced angiogenic activity (IC50=0.8 nM).
There was much excitement earlier this year when, 48 years after its discovery, Fang P. et al. came out with a study describing the mechanism of action of angiogenesis inhibitor Borrelidin. (1)
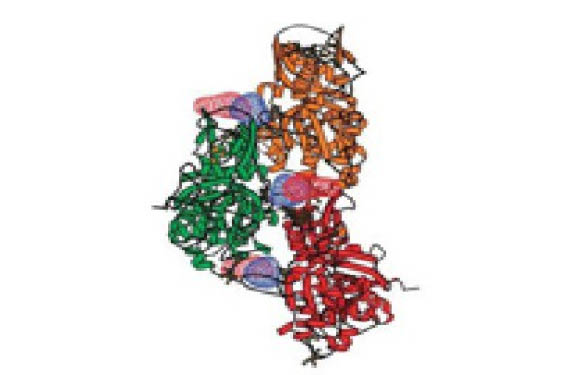
Pr. Min Guo (Scripps Research Institute in Florida) has uncovered the details of its unique mechanism of binding and inhibition.
Using X-ray crystallography, Pr. Guo and his co-workers showed that Borrelidin simultaneously binds to four distinct sites in the catalytic domain of ThrRS including the three substrate binding sites for ATP, aminoacid and tRNA as well as a fourth orthogonal site created as a consequence of binding. This surprising natural design results in a potent and redundant mechanism for quadrivalent inhibition of ThrRS during protein synthesis.
Its unique mechanism of action together with its high ThrRS inibiting activity make Borrelidin a promising candidate for therapeutic applications and research programs related to infectious and immune diseases as well as oncology. For this latter purpose, Focus Biomolecules supplies stock quantities (Fermentation product from Streptomyces sp. – 1 mg) as well as bulk of Borrelidin for research use only (>98% by HPLC).
References:
(1) Fang P. et al. “Structural basis for full-spectrum inhibition of translational functions on a tRNA synthetase” (2015) Nature Communications; 6:6402. DOI: 10.1038/ncomms7402.