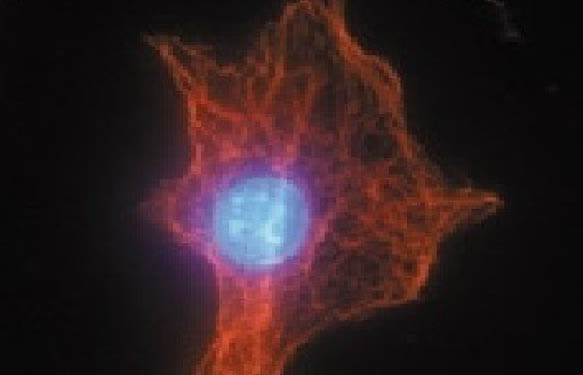
Recently, B.-H. Choi et al. characterized a novel anti-mitotic molecule termed suprafenacine which destabilizes microtubules, resulting in cell cycle arrest in the G2/M phase and apoptotic cell death. In silico screening identified several novel anti-cancer molecules based on their ability to inhibit in vitro cell proliferation and tubulin  polymerization. Structure Activity Relationship studies guided the synthesis of several analogues. Of these analogues, suprafenacine was the most potent based on its in vitro ability to 1. specifically target cancer cells from multiple tumor types and 2. inhibit tubulin polymerization (IC50 = 0.38 mM). Suprafenacine inhibited both the nucleation and growth phases of polymerization, leading to a lower level of steady state polymers. Biotinylated tubulin was used in a tubulin competition binding scintillation proximity assay to demonstrate that the drug binds at the colchicine site (not taxol or vinblastine), but displays a binding mode distinct from colchicine. Thus, this cell-permeable, microtubule-destabilizing anti-cancer drug can serve as a template for designing and synthesizing future anti-cancer drugs.
polymerization. Structure Activity Relationship studies guided the synthesis of several analogues. Of these analogues, suprafenacine was the most potent based on its in vitro ability to 1. specifically target cancer cells from multiple tumor types and 2. inhibit tubulin polymerization (IC50 = 0.38 mM). Suprafenacine inhibited both the nucleation and growth phases of polymerization, leading to a lower level of steady state polymers. Biotinylated tubulin was used in a tubulin competition binding scintillation proximity assay to demonstrate that the drug binds at the colchicine site (not taxol or vinblastine), but displays a binding mode distinct from colchicine. Thus, this cell-permeable, microtubule-destabilizing anti-cancer drug can serve as a template for designing and synthesizing future anti-cancer drugs.
The results published in this paper were achieved with kits produced by Cytoskeleton Inc.. Their Fluorescent tubulin polymerization assay kit and biotinylated tubulin were essential in this study, providing in vitro characterization of suprafenacine’s effects on tubulin polymerization and revealing the drug’s tubulin binding site. These kits are available in Europe through their long-standing distributor tebu-bio.
References:
Interested in getting more information about the products used in this study? Leave your comments or ask any questions below!