In a recent post (PARPs as cancer drug targets – first EC-approved drug) I wrote about Olaparib, a drug against ovarian cancer, which has been developed by AstraZeneca and was approved in December 2014 by the European commission – thus being the first drug directed against a member of the PARP family (Poly ADP-Ribose Polymerases). Furthermore I informed about the tools tebu-bio is able to provide to screen for PARP inhibitors, such as active PARP enzymes and ready-to-use inhibitor screening assays for PARP1 , 2, 3, 6, 7, 10, 11, 14, and 15 as well as for Tankyrase 1 and 2.
In this post, let’s take a look at PARP modulators/reference inhibitors especially linked to cancer and Parkinson’s disease research and screening activities.
PARPs and cancer
Poly ADP-ribose polymerases catalyze the synthesis of poly ADP-ribose chains on DNA and protein targets. The involvement of PARP1 and 2 in single strand break rep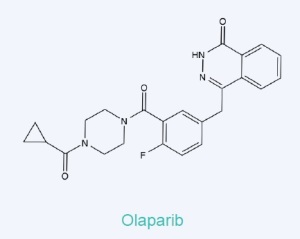 air has made PARPs attractive targets for breast and ovarian cancer1. Rapidly dividing mitotic cancer cells or tumors with mutations in DNA-repair pathways (BRCA1, BRCA2 or PALB2) are sensitive to PARP inhibitors whereas normal cells with intact DNA repair pathways can survive inhibition of PARP. I described this “synthetic lethality” approach in my previous blog post. Interestingly, certain classes of PARP inhibitors can trap PARP proteins on damaged DNA, a novel mechanism of action that leads to apoptosis distinct from inhibiting PARP catalytic activity.
air has made PARPs attractive targets for breast and ovarian cancer1. Rapidly dividing mitotic cancer cells or tumors with mutations in DNA-repair pathways (BRCA1, BRCA2 or PALB2) are sensitive to PARP inhibitors whereas normal cells with intact DNA repair pathways can survive inhibition of PARP. I described this “synthetic lethality” approach in my previous blog post. Interestingly, certain classes of PARP inhibitors can trap PARP proteins on damaged DNA, a novel mechanism of action that leads to apoptosis distinct from inhibiting PARP catalytic activity.
The most prominent inhibitor affecting PARP activity is Olaparib, which I already mentioned as the first drug targeted against PARPs which has been approved.
Nevertheless, several compounds have been described as being linked to PARP inhibition and cancer – you might like to take a look at an overview of these Cancer related PARP inhibitors.
PARPs and Parkinson’s disease
The inactivation of the ubiquitin E3 ligase parkin appears to be a pathogenic feature in both familial and sporadic Parkinson’s disease. Under normal circumstances, parkin-mediated K48 ubiquitinyla tion of substrate proteins such as AIMP2 leads to the substrate proteins being degraded by the ubiquitin/proteasome pathway. When parkin activity is absent or reduced, these substrate proteins accumulate in the cell and in the case of AIMP2 cause degradation of dopaminergic neurons. Studies have shown that AIMP2 accumulation in vitro and in vivo leads to PARP1 overactivation, and to a Parkinson’s disease-like selective and progressive age-dependent loss of dopaminergic neurons that is PARP dependent. Inhibition of PARP through gene deletion, or by small molecule inhibitors, protects against the dopamine neuron death, making PARP an interesting target for new, brain permeable drugs that could possibly delay or prevent disease progression in patients with Parkinson’s disease.
tion of substrate proteins such as AIMP2 leads to the substrate proteins being degraded by the ubiquitin/proteasome pathway. When parkin activity is absent or reduced, these substrate proteins accumulate in the cell and in the case of AIMP2 cause degradation of dopaminergic neurons. Studies have shown that AIMP2 accumulation in vitro and in vivo leads to PARP1 overactivation, and to a Parkinson’s disease-like selective and progressive age-dependent loss of dopaminergic neurons that is PARP dependent. Inhibition of PARP through gene deletion, or by small molecule inhibitors, protects against the dopamine neuron death, making PARP an interesting target for new, brain permeable drugs that could possibly delay or prevent disease progression in patients with Parkinson’s disease.
You’ll find here an overview of PARP inhibitors linked to this focus of neurogenerative disease research: Parkinson’s disease related PARP inhibitors.
And finally, here’s an overview of all PARP modulators available at tebu-bio.com.
Interested in PARP modulators for your research? As reference compounds for your screening activities? Get in touch with me through the form below!



