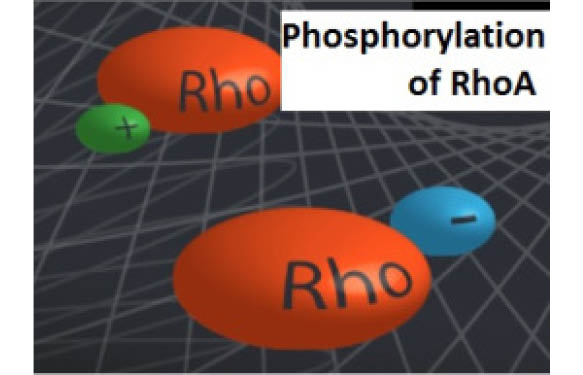
Rho family GTPases are key regulators in a wide range of physiological processes, including cell motility, cell division, and neuronal development. Rho activity is regulated temporally and spatially by a variety of direct post-translational modifications (PTMs) that include prenylation, ubiquitination, oxidation, nitrosylation, and phosphorylation.
Cytoskeleton Inc. recently released a newsletter highlighting the control of RhoA function through phosphorylation. RhoA is a target for a growing number of kinases and as such, phosphorylation is emerging as a central theme in the regulation of this family of proteins (2).
The newsletter focussed on the mechanism of RhoA phosphorylation at Serine 188, which is mainly conducted by kinases like PKA and PKG (protein kinase A and protein kinase G) which are cyclic AMP-dependent and cyclic GMP-dependent respectively.
Furthermore, it looks at the physiological consequences of RhoA phosphorylation and future directions especially concerning the RhoA PTM involvement in diseases and potential therapeutic options.
You can download a copy of this newsletter, or if you have any questions or comments, don’t hesitate to contact me through the form below.
Related to RhoA and PTM research:
- G-LISA kits to measure the activation of RhoA
- Cell permeable RhoA inhibitor (C3Transferase)
- RhoA activators
- Anti Acetyl Lysine Mouse Monoclonal Antibody
- Anti-SUMO1 Mouse Monoclonal Antibody
- Anti-Ubiquitin Mouse Monoclonal Antibody
References:
1. Stankiewicz T. & Linseman D. 2014. Rho family GTPases: key players in neuronal development, neuronal survival and neurodegeneration. Front. Cell. Neurosci. doi: 10.3389/fncel.2014.00314.
2. Boulter E. et al. 2012. Off the beaten paths: alternative and crosstalk regulation of Rho GTPases. FASEB J. 26, 469-479.