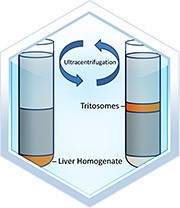
Rat liver tritosomes are hepatic lysosomes that have been loaded with Tyloxapol (Triton WR 1339), a non-ionic surfactant. Tyloxapol is taken up by hepatocytes through endocytosis and is trafficked to lysosomal compartments. Tyloxapol containing lysosomes exhibit decreased density and can be more efficiently isolated away from contaminating cellular organelles that have overlapping densities with native lysosomes (1-3).
 The use of tritosomes is well-established and has been classically used to study lysosomal composition, function, and disease (4-6). More recently they have become a powerful reagent to assess the in vitro stability and release of small molecular moieties conjugated to nano particles, polymers and antibodies that enter cells through the endosome-lysosome pathway (7-10).
The use of tritosomes is well-established and has been classically used to study lysosomal composition, function, and disease (4-6). More recently they have become a powerful reagent to assess the in vitro stability and release of small molecular moieties conjugated to nano particles, polymers and antibodies that enter cells through the endosome-lysosome pathway (7-10).
Features and benefits of Rat Liver Tritosomes
- Highly purified
- Characterized for lysosome specific enzymatic activity
- Less complex than in vivo models
- More representative than using individually expressed/purified enzymes
Interested? Discover Mixed gender IGS SD Rat Liver Tritosomes, available across Europe through tebu-bio.com
References
- Leighton, F., Poole, B., Beaufay, H., Baudhuin, P., Coffey, J. W., Fowler, S., and De Duve, C. (1968) The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. The Journal of cell biology 37, 482-513
- Trouet, A. (1974) Isolation of modified liver lysosomes. Methods in enzymology 31, 323-329
- Hayashi, H., Niinobe, S., Matsumoto, Y., and Suga, T. (1981) Effects of Triton WR-1339 on lipoprotein lipolytic activity and lipid content of rat liver lysosomes. Journal of biochemistry 89, 573-579
- Maguire, G. A., Docherty, K., and Hales, C. N. (1983) Sugar transport in rat liver lysosomes. Direct demonstration by using labelled sugars. The Biochemical journal 212, 211-218
- Lubke, T., Lobel, P., and Sleat, D. E. (2009) Proteomics of the lysosome. Biochimica et biophysica acta 1793, 625-635
- Della Valle, M. C., Sleat, D. E., Zheng, H., Moore, D. F., Jadot, M., and Lobel, P. (2011) Classification of subcellular location by comparative proteomic analysis of native and density-shifted lysosomes. Molecular & cellular proteomics : MCP 10, M110 006403
- Hreczuk-Hirst, D., Chicco, D., German, L., and Duncan, R. (2001) Dextrins as potential carriers for drug targeting: tailored rates of dextrin degradation by introduction of pendant groups. International journal of pharmaceutics 230, 57-66
- Malugin, A., Kopeckova, P., and Kopecek, J. (2007) Liberation of doxorubicin from HPMA copolymer conjugate is essential for the induction of cell cycle arrest and nuclear fragmentation in ovarian carcinoma cells. Journal of controlled release : official journal of the Controlled Release Society 124, 6-10
- Lim, E. K., Jang, E., Lee, K., Haam, S., and Huh, Y. M. (2013) Delivery of cancer therapeutics using nanotechnology. Pharmaceutics 5, 294-317
- Barrett, S. E., Abrams, M. T., Burke, R., Carr, B. A., Crocker, L. S., Garbaccio, R. M., Howell, B. J., Kemp, E. A., Kowtoniuk, R. A., Latham, A. H., Leander, K. R., Leone, A. M., Patel, M., Pechenov, S., Pudvah, N. T., Riley, S., Sepp-Lorenzino, L., Walsh, E. S., Williams, J. M., and Colletti, S. L. (2014) An in vivo evaluation of amphiphilic, biodegradable peptide copolymers as siRNA delivery agents. International journal of pharmaceutics 466, 58-6