Since its emergence in 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread to become the major health issue of the past two years. But it’s also a research field of interest for many researchers. With the latest findings, SARS-CoV-2 has more to reveal… including one related to ADCC.
Anti-SARS-CoV-2 Ab mediates ADCC
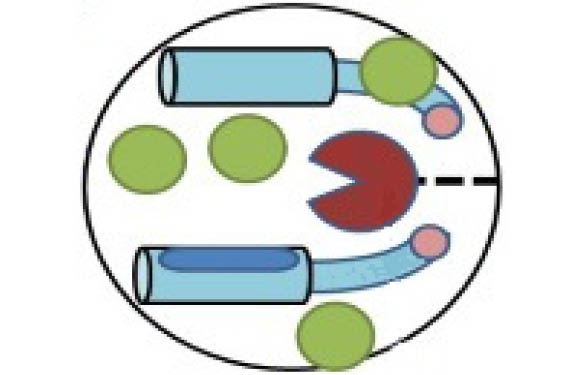
ADCC is a well-known and important mechanism for the immune system to target diseased or infected cells. It’s a reaction whereby immune cells are able to carry out cytolysis via antibodies that “designate” the cells to be lysed (Fig. 1). Neutralizing-antibody (nAb) is the major focus of most ongoing COVID-19 vaccine trials. However, nAb response against SARS-CoV-2, when present, decays rapidly. Interestingly, recent clinical evidence indicates that SARS-CoV-2 infected patients develop both nAb and non-nAb mediating ADCC, which is predicted to be a positive surrogate of clinical remission (Tso FY, Lidenge SJ, Poppe LK, and al. (2021)).
A new ADCC Screening service to advance your SARS-CoV-2 research
In response to this recent evidence, tebu-bio’s laboratory experts have developed an ADCC screening assay, allowing you to quickly reveal the potential of your antibodies in a SARS-CoV-2 context.
Assay Principle
The ADCC screening assay developed by tebu-bio’s experts is based on the use of two engineered cell lines:
- Effector cells: Jurkat cells expressing Fc receptors and inducible firefly luciferase (developed by BPS Bioscience)
- Target cells : HEK293T cells expressing SARS-Cov-2 Spike Glycoprotein.
The Jurkat T effector cells contain a firefly luciferase reporter gene under the control of the NFAT response element. In addition, the cells express constitutively the human receptor FcγRIIIa, high affinity (V158) variant and Fcγ chain. Upon Fc receptor activation, NFAT-dependent signaling activates the expression of luciferase. The HEK engeneered cells are constituvely expressing the SARS-CoV-2 Spike Glycoprotein.
The Spike-HEK target cells are co-cultured with Jurkat effector cells, in the presence or absence of a test antibody. If the test antibody displays ADCC-inducer activity, the compex [target cell : ADCC-inducer Ab : effector cell] formation induce the activation of luciferase expression that can be monitored using luminescent revelation reaction (Fig. 2).

Screening results
Our ADCC screening service offers you the possibility to determine the efficacy of your sample in mediating ADCC towards SARS-CoV-2. Our lab experts can evaluate this ADCC efficiency from your antibodies or directly from your serum sample.
Step 1 : Eliminate aspecfic ADCC effect
The first step is to co-incubate the Effector cells with the antibody in the absence of target cells, to evaluate the natural ADCC-independant noise signal (Fig. 3).
Step 2 : Assess real ADCC efficiency
Thus, your sample is incubated at increasing concentrations with both target and effector cells to determine ADCC efficiency (fig. 3).
The final, real ADCC efficiency evaluation of your sample will be based on the substraction of the ADCC-independent noise signal and conversion into fold induction (Fig. 4). This normalization is our guarantee to provide you with accurate and unbiased results.
Your challenges – Our solutions
In answer to your challenges of revealing the ADCC efficiency of your compound, we enable you to:
- Select and Rank the most promising antibodides based on their ADCC efficiency
- Determine the IC50 or single point concentration analysis for all your hits
- Combine tebu-bio’s SARS-CoV-2 screening service with our other services, to determine the global MoA of your antibodies (e.g. ADCC and/or only blocking effect…).
The development of ADCC-promoting Ab represents a new route to the development of anti-COVID-19 treatments. Let us help you to speed up your drug development program with our new ADCC screening assay!
Do you have any specific assay development requirements? Ask our lab experts to develop the assay you need!