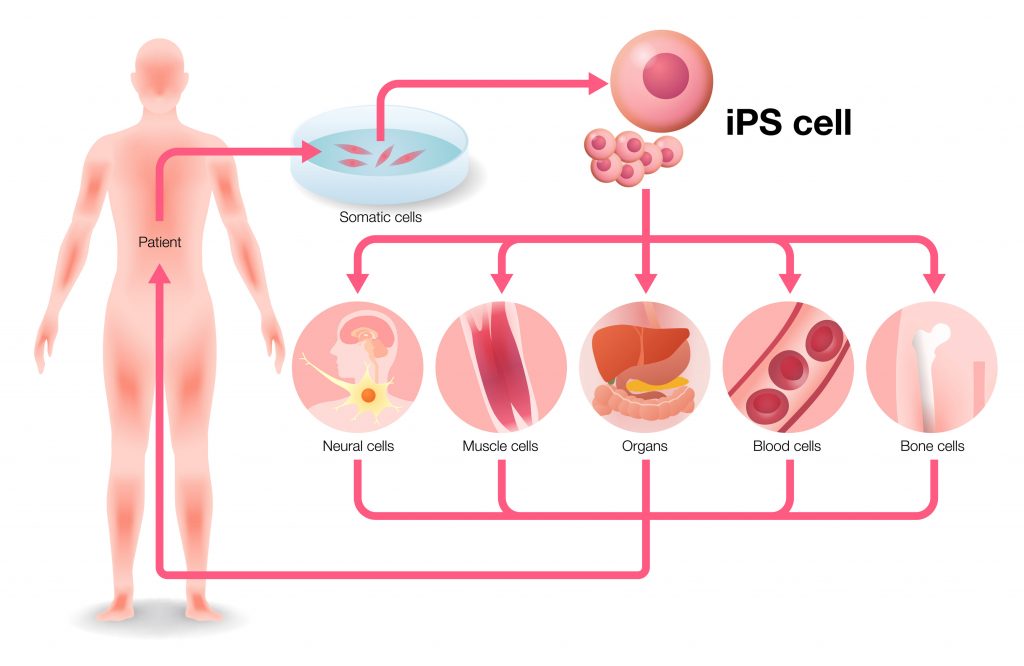
In collaboration with our partner Ajinomoto, recognised amino-acid experts, we are launching in Europe a new Xeno-free, defined medium for Human Embryonic Stem cells and induced Pluripotent Stem cell (iPS) culture. The new StemFit® Basic02 is a high performance single expansion medium for Stem cell and iPS cell weekend free cell culture.
You might be thinking that there are already quite a few media on the market for iPSC culture, so here I’d like to emphasize the specific features that make StemFit® Basic02 unique:
- Superior and stable growth performance
- High colony forming efficiency
- Robust, scalable cell expansion
- Weekend free feeding – 3 media changes per week
- Low volume cell culture
StemFit® for superior growth performances & high colony forming efficiency from single cell expansion
StemFit® Basic02 was co-developed with S. Yamanaka (Nobel prize in 2012 for his work on cell reprogramming discovery), using a defined amino-acid composition. It is already widely used in Japan.
The ability of this medium to support superior growth performances and high colony forming efficiency from single cell expansion (particularly useful in CRISPR strategies) has already been demonstrated in highly ranked journals such as Nature and Stem Cell reports. You can browse a selection of articles I have made for you at the end of this article.
The low lactate accumulation overtime that has been demonstrated may be one the factors responsible for the robust genome stability observed and superior growth under weekend-free feeding conditions.
StemFit® iPS / Stem cell expansion medium in action
Robust and reproducible cell expansion of StemFit® was validated by a third party at Cell and Gene Therapy (CGT) – Catapult.
In this experiment, RCiB10 iPSCs (Clinical grade iPSC line) grew at higher rate when cultured in StemFit® without karyotype abnormality. If you’d like to explore their findings in more detail, you can download the poster “Superior performance of StemFit® AKN-03 for the culture of induced pluripotent stem cells” by CGT Catapult here.
This unusually high growth performance was illustrated after a 7 days culture on several matrices; all showed a very high fold expansion when compared to standard iPSC medium on the market:

As the medium requires less volume per well (eg. 1.5ml in a well for a 6-well plate while standard recommendations are 2ml), and as the number of medium change are lower, this higher performance is also cost and time effective.
An example below of a possible media change time table demonstrates the flexibility of the culture, including weekend free conditions:

StemFit® for single iPS / Stem cell passage
I indicated earlier that an efficient single cell passage was one of the strong benefits provided by StemFit, a benefit of most importance at the cloning stage of iPSC cell line generation by CRISPR strategies. The few tips below provided by the manufacturer will help you get optimal results for this cell line generation step:

Finally, if the data above have tempted you to try this media for your iPSC culture, the tips below will help you switch from your previous medium to this one:

The medium does not include bFGF. We recommend to start by adding a high concentration during the first few days of culture (100ng/ml) and carry on with your usual concentration depending on your cell type (between 4 to 100ng/ml).
Interested in single iPS / Stem cell expansion?
Please feel free to contact me if you’re interested in transitioning to this new media for improving your induced Pluripotent Stem cell culture performance, either via a comment below or through the “contact us” button at the top of this screen – I’ll be happy to discuss this with you!
Citations using the StemFit® high performance iPS / Stem cell medium
1 – A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells – Masato Nakagawa et al, Scientific Reports 4:3594 (2014)
2 – Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells – Ryuji Morizane & Joseph V Bonventre, Nature Protocols Vol 12 No 1 (2017)
3 – Engineering the AAVS1 locus for consistent and scalable transgeneexpression in human iPSCs and their differentiated derivatives – F. Oceguera-Yanez et al., Methods (2015)
4 – Establishment of In Vitro FUS-Associated Familial Amyotrophic Lateral Sclerosis Model Using Human Induced Pluripotent Stem Cells – Naoki Ichiyanagi et al, Stem Cell Reports, Vol 6 (2016)
5 – Genetic and pharmacological correction of aberrant dopamine synthesis using patient iPSCs with BH4 metabolism disorders – Taizo Ishikawa et al, Human Molecular Genetic (2016)
6 – Multilineage communication regulates human liver bud development from pluripotency – J. Gray Camp et al, Nature Letter doi:10.1038/nature22796 (2017)
7 – Co-ordinated ocular development from human iPS cells and recovery of corneal function – Ryuhei Hayashi et al, Nature, Vol 531 doi:10.1038/nature17000 (2016)
8 – Derivation and differentiation of haploid human embryonic stem cells – Ido Sagi et al, Nature Letter doi:10.1038/nature17408 (2016)
Additional publication:
- Takahashi K, Yamanaka S. “Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors” Cell. 2006 Aug 25;126(4):663-76. Epub 2006 Aug 10.