To better understand the mechanism of SARS-CoV-2 cell entry, BPS Bioscience have developed 3 new pseudotyped lentiviruses, allowing you to study the interaction between the coronavirus Spike protein and the human ACE2 protein in a physiologically relevant context.
SARS-CoV-2 interacts and penetrates into human cells through the binding of the Spike protein localized out of it’s membrane and ACE2 (angiotensin converting enzyme 2), a homodimer receptor found on the surface of some human cells, particularly those in the respiratory tract. The Spike protein, which consists of two subunit (S1 and S2) (1), promotes attachment to ACE2 thanks to the subunit S1 and its RBD (Receptor Binding Domain) region. After enzymatic cleavage by cellular proteases (furin and TMPRSS2) the S2 subunit facilitates fusion of the viral and cellular membrane allowing the virion to enter the cell (2). Based on this observation, drugs targeting the interaction between the Spike protein of SARS-CoV-2 and ACE2 may offer protection against the viral infection.
BPS Bioscience are actively working to provide Covid-19 research tools to support scientists in their work to find an efficient treatment. They have already developed a series of biochemical assays, to identify inhibitors of the Spike–ACE2 interaction.
Based on the sandwich ELISA technique (fig.1), these 96 well plate assays allow quick screening and identification of compounds (chemicals or antibodies) able to block the Spike:ACE2 interaction and virus penetration. You can find more information on these assays in our previous post : “New SARS-CoV/ACE2 inhibitor assays & small molecules for drug discovery“.
To go further with these screening tools, BPS Bioscience have just launched three new pseudoviruses on the market to build your own cell based assay and validate the neutralizing effect of compounds on the Spike:ACE2 binding in a more physiological context.
1 – ACE2 Lentivirus
Localized on the surface of many human cell types (lungs, arteries, heart, kidney, and intestines) ACE2 is the entry point of the virus due to its interaction with the coronavirus membrane protein: Spike.
The ACE2 Lentivirus are replication incompetent, HIV-based, VSV-G pseudotyped lentiviral particles that are ready to be transduced into almost all types of mammalian cells, including primary and non-dividing cells. Under the control of EF1a promoter (fig. 2) the particles contain an ACE2 gene (NM_021804.3) allowing a transient expression of ACE2 in your target cell or a generation of a stable cell line expressing ACE2 with Puromycin.

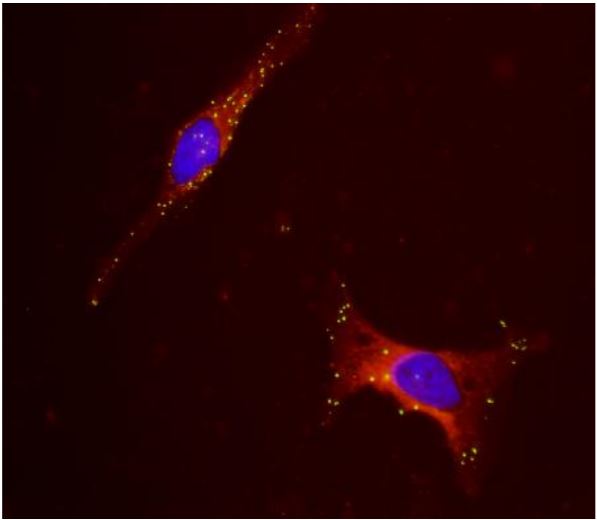
The transduction efficiency and the expression of ACE2 in HEK293 cells has been evaluated by FACS analysis (fig. 3) with two different strategies. The first used a specific anti-human ACE2 antibody (fig. 3 A). The transduced cells were stained by anti-human ACE2 polyclonal goat IgG primary antibody and Alexa Fluor 488-conjugated rabbit anti-goat IgG as a secondary antibody. In the second strategies (fig. 3b) the transduced cells were stained by a specific ACE2 ligand (A biotinylated Spike S1 protein) and the Phycoerythrin (PE) conjugated to Streptavidin for the detection. In both cases FACS analysis reveal a high level of ACE2 expression at the surface of the HEK293 cells.
2 – Spike (SARS-CoV-2) Pseudotyped Lentivirus (Luciferase Reporter)
Due to its interaction with the Human ACE2 , the coronavirus Spike protein is involved in the first step of the viral replication who is the attachment of the virus to the host cell. In combination to the ACE2 lentivirus, BPS Bioscience have developed the Spike (SARS-CoV-2) Pseudotyped Lentivirus.
Here, the commonly used VSV-G viral fusion protein, which classically mediates the fusion between the virus envelope and host cellular membrane, has been replaced by the SARS-CoV-2 Spike protein
(Genbank Accession #QHD43416.1) as the envelope glycoproteins. These pseudovirions also contain the firefly luciferase gene driven by a CMV
promoter (fig. 4), to easily measure the spike-mediated cell entry via luciferase reporter activity (Detection performed with the optimized ONE-Step Luciferase reagent ).
Transduction efficiency of the Spike (SARS-CoV-2 ) pseudotyped lentivirus has been evaluated on HEK293 cells expressing or not the ACE2 protein (fig. 5A) and on Calu3 cells (human lung cancer cell line which are epithelial and can act as respiratory models in preclinical applications) (fig. 5B) used as a transduction control. Based on Luciferase measurement it appears that the Spike (SARS-CoV-2) pseudotyped lentivirus transduced ACE2-HEK293 and Calu3 cells with much greater efficiency compared with HEK293 parental cells. It indicates that the transduction is dependent upon ACE2 expression. The bald lentiviral pseudovirion, where no envelope glycoprotein is expressed, was used as a negative control.

Spike (SARS-CoV-2) pseudotyped lentivirus.
With ACE2 dependent transduction efficiency proved, several neutralization assays have been performed with an anti-SARS-CoV-2 Spike antibody (fig. 6), a recombinant ACE2 protein (fig. 7) and an anti-ACE2 antibody (fig. 8).

For the three neutralizing assays, the HEK293 expressinng ACE2, were transduced with a mix containing the Spike (SARS-CoV-2) pseudotyped lentivirus and different concentration of the corresponding neutralizing compounds. The % of control transduction corresponds to the luciferase measured in the control wells with the ACE2 expressing cells and the virus, without any neutralizing compounds (fixed at 100% / more details in the data sheet). These three independent experiments showed a lower transduction efficiency of the Spike (SARS-CoV-2) Pseudotyped Lentivirus (decrease in the luciferase expression), due to an inhibitory effect of the tested compounds.

In combination with ACE2 Lentivirus, this Spike (SARS-CoV-2) pseudotyped lentivirus can be used to study the mechanism of viral transduction, and easily screen for neutralizing compounds (antibodies – chemical compounds) of the SARS-CoV-2 Spike and ACE2 interaction, in a Biosafety Level 2 facility.
3 – Bald Lentiviral Pseudovirion (Luciferase Reporter)
The bald lentiviral pseudovirion was produced without envelope glycoproteins such as VSV-G or SARS-CoV-2 spike. It contains the firefly luciferase gene driven by a CMV promoter as the reporter (fig. 9). The bald lentiviral pseudovirion can serve as a negative control when studying virus entry initiated by specific interactions between virus particles and receptors.
Interested in making your own Spike:ACE2 screening cell based assay?
tebu-bio not only supports researchers by providing innovating reagents and research tools, but also by allowing them to gain time thanks to our laboratory services. In fact, our laboratory has developed many services to help you in evaluating your current drug portfolio or lead NMEs for anti-COVID-19 therapeutic purposes, but also many other research fields.
Interested in testing your portfolio on our SARS-CoV-2/ACE2 in vitro screening assays? Or maybe directly in physiologically relevant context thanks to these new pseudovirus tools ?
References:
- Ou, X., et al. 2020. Nat Commun 11: 1620. doi:10.1038/s41467-020-15562-9
- Yan, R., et al. BioRxiv. 2020 Feb 18; in press. doi:10.1101/2020.02.17.951848







2 responses
Hello Contacted regarding COVID batch release testing tools. Some one has called us. Unfortunately, the phone number was withheld that we could not call back.
Please send us an email with contact details.
Dear Chennakesava,
Thank you for your message.
My colleagues from our Germany office (also taking care of our customers from Switzerland) can be contacted at:
germany@tebu-bio.com; Tel: (069) 801013-0.
I will let them know that you are trying to reach them anyway.
Kind regards,
Isabelle