Generating In Vivo Relevant Transporter Data:
Using Transporter Certified™ Cryopreserved Hepatocytes
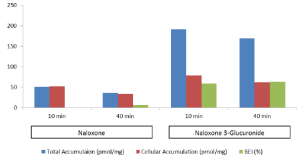
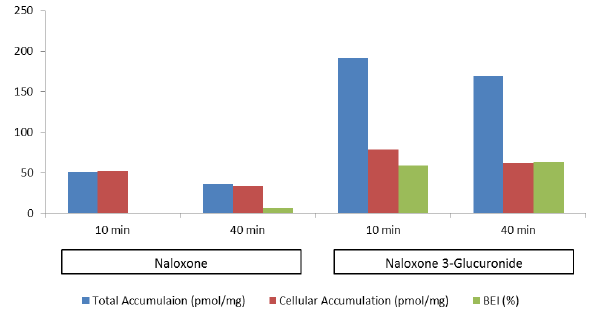
This recent webinar highlighted the processes that are involved in the certification of cryopreserved human hepatocytes for transporter function and the advantages in using them to characterize a compound’s hepatobiliary disposition, transporter interaction potential and overall hepatic clearance.
We discussed how Transporter Certified™ hepatocytes can work with a B-CLEAR® Cell-Less kit to provide in vivo relevant transporter information.
Interested in catching up on this webinar?
Contact jean-francois.tetu@tebu-bio.com to receive the link to the recorded webinar or the slides that were presented.
Some further reading – the posts below are on related topics of interest:
- in vitro exploration of hepatobiliary drug disposition
- Maximise availability & reduce variability in hepatocytes studies
- 6 tips for thawing hepatocytes