DNA topoisomerase I and DNA topoisomerase II (Topo I and Topo II) are nuclear enzymes which regulate the topological state of the DNA helix by transiently breaking and rejoining DNA strands. They also play a critical role in fixing DNA damage resulting from an exposure to harmful chemicals or UV rays. Topo small molecule inhibitors are well-known anticancer approaches. Today, they are coming back to the Drug discovery scene just enough to edit a post on Topo inhibitors, from their history and to their availability for in vitro R&D purposes.
While Topo I cuts single stranded DNA double helix, Topo II cleaves both DNA strands of DNA through an ATP dependant reaction. Both enzymes are then crucial in maintaining genomic integrity of eukaryotic cells during major cellular processes (replication, transcription, recombination, chromatin remodeling…).
Topo inhibitors: let’s go back to the sixties
In the 60s, the ability to interfere with Topo enzymes via small molecule inhibitors (sometimes called Topo poisons) became an effective strategy in oncology. DNA Topos rapidly became excellent druggable targets of clinically significant classes of anticancer drugs (ex. ovarian, lung, colon cancers) by using them as cytotoxic agents to kill cells undergoing DNA replication or repair, and reading of the DNA for transcription.
The idea was to capitalize on cancer cells over proliferation (compared to normal cells’ proliferation rate), and kill mainly cancer cells by Topo inhibitors (though some normal cells with topoisomerase activity might also be killed).
Topo inhibitors’ action mode is based on their capacity to bind to the Topo enzyme making it inactive. By blocking Topo enzyme’s ability to bind DNA after it has been cut, DNA is never repaired leading to cell death.
Several limitations were rapidly identified with the first generation of Topo inhibitors: toxicity and low poor water solubility (ex. Camptothecin Topo I inhibitor and severe urological and gastric toxicity).
Topo inhibitor analogs are now making a comeback on the clinical field with the development of new analogs showing higher solubility (and thus making administration easier) and limited organ toxicity and Adverse Drug Reaction.
In parallel, these analogs are described as promising active molecules for cancer treatment (combination chemotherapy regimens) but also in other medical areas like infectious diseases (ex. antiparasitic effects on Trypanosoma, antiviral chemotherapy on Herpesvirus).
Which DNA Topoisomerases analogs for R&D purposes
Accessing high purity and cell permeable Topo inhibitors is sometimes tricky. Among them, 5 bioactive small molecules can be highlighted:
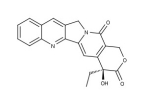
#1- Camptothecin (CPT)
A cytotoxic quinoline alkaloid discovered by systematic screening of natural products for anticancer drugs. This natural compound (Camptotheca acuminate chinese Happy tree) is active on DNA topoisomerase I. CPT, an FDA approved anticancer drug, binds irreversibly to the topoisomerase I /DNA complex preventing DNA re-ligation generating permanent double strand breaks.
#2- Irinotecan (Camptothecin-11, CPT-11)
A semi-synthetic derivative of Camptothecin. This prodrug is converted by tissue Esterases to 7-ethyl-10-hydroxycamptothecin (SN-38), a potent inhibitor of DNA topoisomerase I.
#3- SN-38 (7-ethyl-10-hydroxycamptothecin)
An active metabolite of CPT-11 that inhibits DNA topoisomerase I and DNA / RNA synthesis. SN-38 displays potent antitumor activity against a range of human tumour cell lines (including HeLa cells). Although irinotecan is also a topoisomerase inhibitor, SN-38 is approximately 1,000 times as potent in purified enzyme studies. In vitro cytotoxicity assays show much greater variability in potency between the two (2-2,000 fold).
#4- Etoposide
It forms a ternary complex with DNA and topoisomerase II. This chemotherapeutic agent prevents DNA strand re-ligation, causing DNA cleavage. This small molecule blocks the cell cycle in S-phase and G2-phase.
#5- Doxorubicin
The antitumor antibiotic induces DNA damage by intercalation and inhibition of topoisomerase II. This DNA damaging agent induces apoptosis in a variety of cell lines. Doxorubicin is a substrate for the Multidrug Resistance Protein 1 (MDR1)(P-gp / ABCB1 / CD243).
Where to find active Topo inhibitors?
Topo inhibitors can be found from various sources. If you are looking for efficient and very affordable DNA Topo inhibitors and their analogs, I can only recommend you those from our friends at Focus Biomolecules. They are synthetising cutting edge biomolecules for research applications. Interestingly, Focus Biomolecules are flexible enough to provide catalogue inhibitors (2 sizes are frequently available; ex. CPT) but also custom synthesis and chemicals that are not yet commercially available.