

Discover how amyloid-β accelerates cellular senescence in human neural stem cells and explore Dojindo's ready-to-use assay kits for precise SA-β-Gal activity detection, enabling deeper insights into aging and neurodegeneration research.
In this blog, discover three assay kits developed by our partner Dojindo to accurately quantify and detect SA-β-Gal activity, and to explore a broad range of cellular processes linked to senescence.
Impact of Amyloid-β on Neural Stem Cell Senescence and Oxidative Stress
To investigate the effect of the pathogenic protein amyloid-β (Aβ) on the senescence of human neural stem cells (NSCs), Dr. Li et.al. performed various analyses related to cellular senescence of human NSCs. Senescent phenotypes are significantly increased and accumulated in cell types (microglia, neuron, and so on) associated with Alzheimer’s disease (AD), age-related neurodegenerative disease, and it affects neurogenesis and cognitive function. In this article, we introduce a report that Aβ accelerates cellular senescence by downregulating the expression of SIRT1 protein in human NSCs.
The analysis of cellular senescence was performed by using indicators such as the expression of cell cycle-related genes p16 and p21, senescence-associated β-galactosidase (SA-β-gal) activity, and activation of DNA damage response (expression of γH2AX). SA-β-gal activity was measured by using a fluorescent probe “SPiDER-βGal”. In human NSCs treated with Aβ, it was found that Aβ significantly increased SA-β-gal activity and the expression of p16 and p21. In addition, it was found that Aβ impairs the mitochondrial respiratory chain complexes, resulting in subsequent ROS generation by confirming the increase of mitochondrial ROS and intracellular ROS levels. This suggests that Aβ causes DNA damage by accumulating ROS and accelerated cellular senescence.
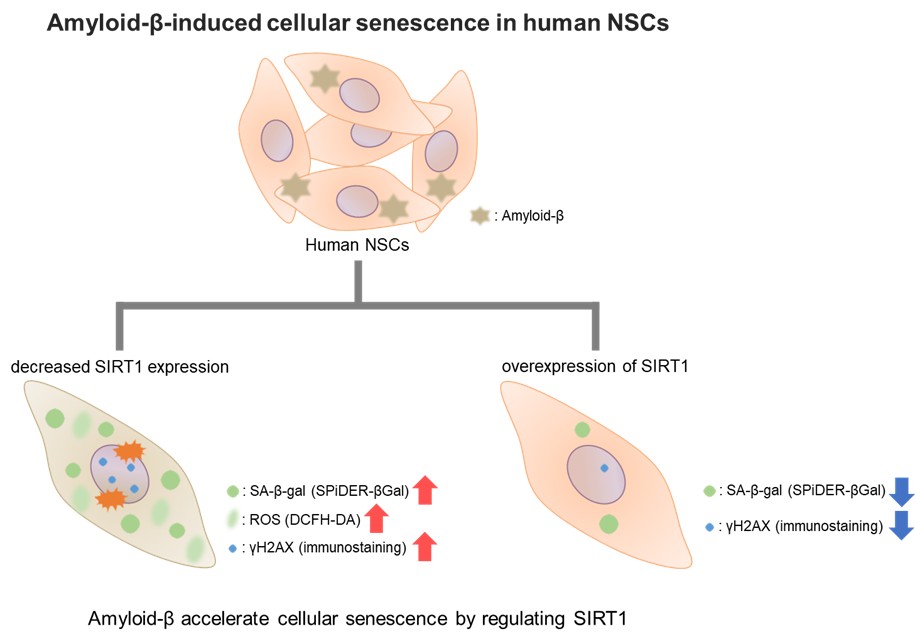
Moreover, Aβ treatment reduced the expression of SIRT1 protein, which affects the cellular senescence. When Aβ treatment was performed in human NSCs overexpressed SIRT1, SA-β-gal activity was significantly decreased, and the expression of γH2AX, p16, and p21 was decreased. This suggests that Aβ accelerates cellular senescence by regulating SIRT1. This result also indicated that SIRT1 overexpression may be able to significantly downregulate Aβ-induced cellular senescence in human NSCs.
This report reveals the effect of Aβ on human NSCs senescence, which is rarely studied. These findings suggest that SIRT1 is a highly promising therapeutic target for anti-aging in human NSCs. Accordingly, studying the treatment strategies for age-related neurodegenerative disorders such as Alzheimer’s disease will continue to develop.
A complete toolbox to assess Cellular Senescence
Cellular senescence has recently gained significant attention across various fields, including stem cell research. This complex biological process involves multiple events such as cell death, metabolic changes, and autophagy.
Specific markers and related activities, such as senescence-associated β-galactosidase (SA-β-gal) activity, can be studied to evaluate cellular senescence and the therapeutic effects of different treatments.
Our partner, Dojindo, has developed efficient, ready-to-use solutions to detect and quantify SA-β-gal activity across a wide range of research models.
SPIDER B-Gal for Precise Quantification:
SPIDER B-Gal is available in two different formats:
- Cellular Senescence Detection Kit [link]— compatible with microscopy and flow cytometry
- Cellular Senescence Plate Assay Kit [link]— compatible with plate readers
Product Highlights:
- Both solutions are suitable for live and fixed cells
- Ideal for SA-β-gal quantification.
- Similar characteristics (Excitation = 500–540 nm; Emission = 530–570 nm)
- A simple 30-minute staining protocol.
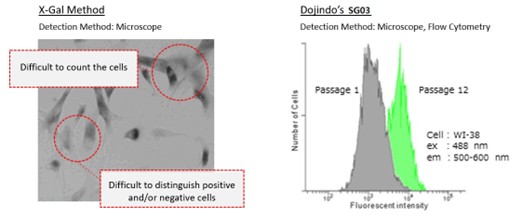
These two kits offer an excellent alternative to the classical X-Gal method, as shown in the image above.
Simple Detection with Spider Blue Probes:
The Cellular Senescence Detection Kit – Spider Blue [link] enables sensitive detection of SA-β-Gal levels and detailed analysis in your research models.
Applicable to fixed cells and compatible with various detection methods (microscopy, flow cytometry, and plate readers), Spider Blue probes (Excitation = 350–450 nm; Emission = 400–500 nm) offer an efficient tool for SA-β-Gal detection.
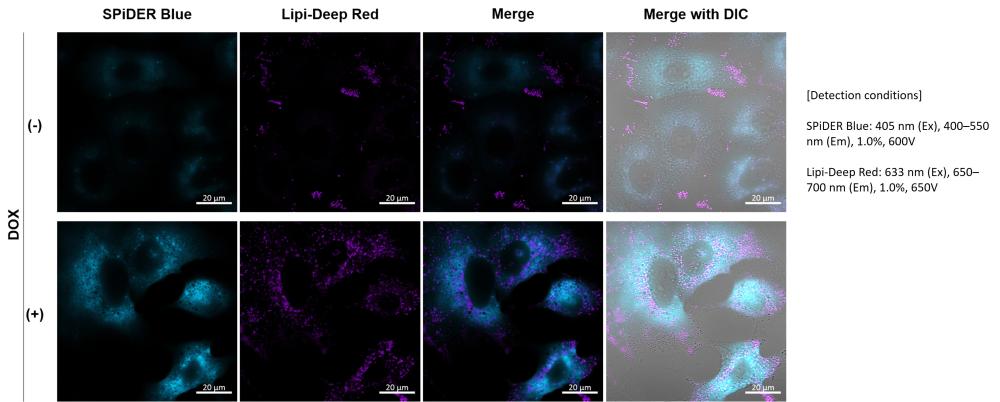
Their compatibility with co-staining and immunostaining (as shown in the image above) also allows for multiparametric analyses of cellular senescence.
Product Highlights:
Cellular Senescence Detection Kit – Spider Blue [link]
- Ready-to-use format for easy and reliable SA-β-Gal detection
- Compatible with microscopy, flow cytometry (FCM), and plate readers
- Suitable for fixed cells
- Enables co-staining and immunostaining for multiparameter studies
- Optimized excitation/emission spectra for flexible experimental setups
Dojindo has developed a full range of ready-to-use assay kits, not only to quantify and detect SA-β-Gal activity but also to explore a wide array of cellular processes associated with senescence — and much more.
-
References
This article has been written Ms. Yasuka Komatsu, Dojindo Laboratories, JAPAN. - Rongyao Li, Yi Li, Haowei Zuo, Gang Pei, Shichao Huang and Yujun Hou, “Alzheimer’s Amyloid-β Accelerates Cell Senescence and Suppresses SIRT1 in Human Neural Stem Cells”, Biomolecules, 2024, 14, 189. doi: 10.3390/biom14020189 - Z. Wang, J. Gao, Y. Ohno, H. Liu and C. Xu, "Rosiglitazone ameliorates senescence and promotes apoptosis in ovarian cancer induced by olaparib.", Cancer Chemother Pharmacol., 2020. - J. H. Cho, E. Kim, Y. Son, D. Lee, Y. S. Park, J. H. Choi, K. Cho, K. Kwon and J. Kim, "CD9 Induces Cellular Senescence and Aggravates Atherosclerotic Plaque Formation.", Cell Death Differ., 2020, doi: 10.1038/s41418-020-0537-9 - A. Kita, Y. Saito, N. Miura, M. Miyajima, S. Yamamoto, T. Sato, T. Yotsuyanagi, M. Fujimiya and T. Chikenji, "Altered regulation of mesenchymal cell senescence in adipose tissue promotes pathological changes associated with diabetic wound healing", Commun. Biol., 2022, doi:10.1038/s42003-022-03266-3. - Y. Haraoka, Y. Akieda, Y. Nagai, C. Mogi and T. Ishitani, "Zebrafish imaging reveals TP53 mutation switching oncogene-induced senescence from suppressor to driver in primary tumorigenesis", Nat. Commun., 2022, doi:10.1038/s41467-022-29061-6. - X. Liu, Z. Liu, Z. Wu, J. Ren, Y. Fan, L. Sun, G. Cao, Y. Niu, B. Zhang, Q. Ji, X Jiang, C. Wang, Q. Wang, Z. Ji, L. Li, C. R. Esteban, K. Yan, W. Li, Y. Cai, S. Wang, A. Zheng, Y. E. Zhang, S. Tan, Y. Cai, M. Song, F. Lu, F. Tang, W. Ji, Q. Zhou, J. Belmonte, W. Zhang, J. Qu, G. Liu, "Resurrection of endogenous retroviruses during aging reinforces senescence", Cell, 2023, doi:10.1016/j.cell.2022.12.017. - Y. Takenaka, I. Inoue, T. Nakano, M. Ikeda and Y. Kakinuma, "Prolonged disturbance of proteostasis induces cellular senescence via temporal mitochondrial dysfunction and subsequent mitochondrial accumulation in human fibroblasts", 2021, doi:10.1111/febs.16249. - H. Yamamoto-Imoto, S. Minami, T. Shioda, Y. Yamashita, S. Sakai, S. Maeda, T. Yamamoto, S. Oki, M. Takashima, T. Yamamuro, K. Yanagawa, R. Edahiro, M. Iwatani, M. So, A. Tokumura, T. Abe, R. Imamura, N. Nonomura, Y. Okada, D. E. Ayer, H. Ogawa, E. Hara, Y. Takabatake, Y. Isaka, S. Nakamura and T. Yoshimori, "Age-associated decline of MondoA drives cellular senescence through impaired autophagy and mitochondrial homeostasis", Cell Rep., 2022, doi:10.1016/j.celrep.2022.110444. - A. Saito, Y. Kamikawa, T. Ito, K. Matsuhisa, M. Kaneko T. Okamoto, T. Yoshimaru, Y. Matsushita, T. Katagiri and K. Imizumi, "p53-independent tumor suppression by cell-cycle arrest via CREB/ATF transcription factor OASIS", 2023, doi:10.1016/j.celrep.2023.112479.
Ready to take to the next step?
From product selection to Contract Research Services, we provide expert guidance tailored to your research project needs.