

An Insight from the Tebubio Team
PARylation is a crucial yet often overlooked post-translational modification linked to DNA repair, cancer progression, and drug response.
This article explores commercial ELISA-based methods that enable researchers to accurately measure PARylation in intact cells, providing valuable insights for drug discovery and biomarker research.
Commercial assays allow researchers to measure this phenomenon in cells
Post-translational modifications—including phosphorylation, methylation, acetylation, ubiquitylation, and glycosylation—are covalent processes that alter the function of proteins.1 PARylation is another post translational modification of emerging interest associated with inflammation, metabolism, and cell death.1
Although PARylation often occurs at very low levels, convenient commercial assays allow researchers to measure this phenomenon in cells.
How PARylation works
PARylation (which stands for poly ADP-ribosylation) involves adding PAR (or poly ADP-ribose) units to proteins in linear or branched forms. This modification is catalyzed by a family of 17 enzymes called PARPs (or PAR polymerases).1 PARPs attach to regions of damaged DNA and then add PARs to themselves (in the process of auto-PARylation) and adjacent nuclear proteins. Ultimately, this henomenon recruits other DNA repair proteins.1
PARylation is reversible and can be undone by PAR erasers, which include PARGs (or PAR glycohydrolases).
PARGs remove the PAR chains and recycle DNA repair proteins to an inactive form.
Researchers have targeted both PARP and PARG pathways for anticancer efforts. For instance, PARP inhibitors (including olaparib, rucaparib, niraparib, and talazoparib) are a class of small molecules that prevent the repair of single-stranded DNA breaks in tumors by inhibiting PARP enzymes,2 leading to the death of cancer cells.
Thus far, PARP inhibitors have been approved to treat ovarian cancer, breast cancer, prostate cancer, and pancreatic cancer.3
Like PARPs, PARGs are overexpressed in many cancers and are associated with tumor growth and survival. PARG inhibitors represent a novel drug class currently being assessed in preclinical and clinical cancer studies.2 Blocking PARG activity leads to PAR buildup on proteins and interferes with DNA repair mechanisms, resulting in the death of cancer cells.4
Measuring PARylation
Physiological PAR levels in cultured cells are challenging to characterize because of low levels and rapid turnover in intact cells.5,6 Various approaches to measure PARylation include radioimmunoassays, radioisotope labeling methods, enzyme-linked immunosorbent assay (ELISA), and stable isotope dilution mass spectrometry.6 However, some of these assays are difficult to perform and cannot measure PAR in intact cells.
Ultimately, PAR levels reflect the balance between PAR writers (e.g., PARPs) and PAR erasers (e.g., PARGS). For example, adding PARG inhibitors pushes the system toward increased PARylation. Conversely, PARP inhibitors decrease protein PARylation.
Commercial PARylation inhibitor mixes
Unlike biochemical assays, cell-based assays allow researchers to evaluate the biological activity of drug candidates in living cells to understand membrane permeability issues and complex interferences.4 Furthermore, adding a drug candidate (such as PARP or PARG inhibitors) to a cell of interest and measuring the resulting levels of PARylation provides information on the compound’s effectiveness in intact cells.
However, physiological levels of PAR are challenging to determine in intact cells because of the rapid synthesis of PAR by PARPs and the breakdown of PAR by PARGs. Further complicating the situation, the random synthesis and degradation of PAR often occurs during cell lysis.
To remedy this problem, some companies sell PARylation inhibitor mixes containing both a PARP inhibitor and a PARG inhibitor.7 These products are designed to prevent alterations in the PARylation states of extracted proteins.
Commercial ELISA-based approaches
Among the various methods for quantifying PARylated proteins, ELISA allows for high sensitivity and the ability to measure many samples simultaneously.6 Commercial PARylation kits typically involve treating cells with PARPs and PARGs of interest and then adding hydrogen peroxide to induce DNA damage. The reaction is then stopped, cell extracts are collected, and lysates are analyzed.
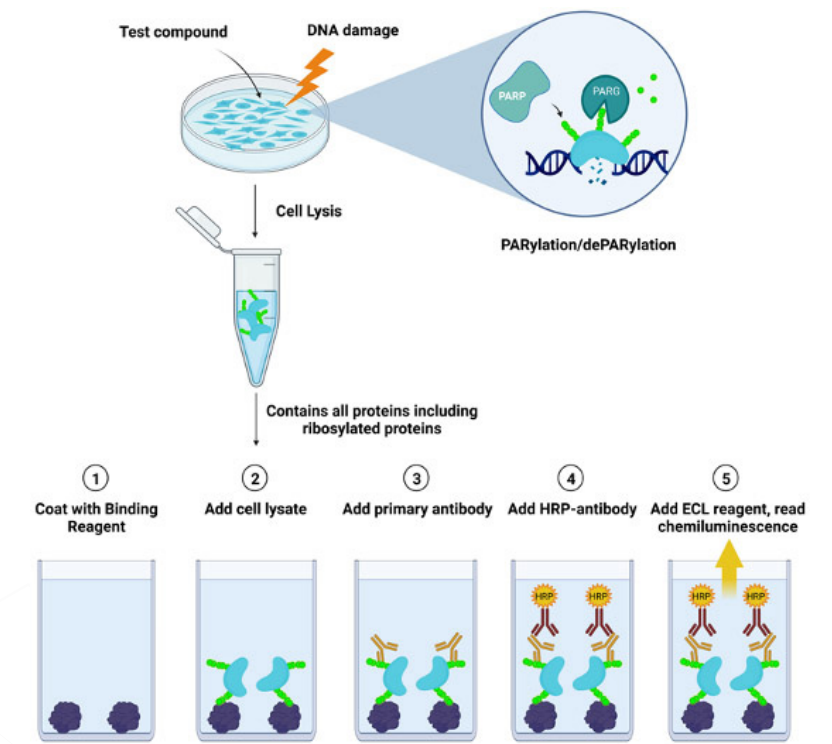
Figure 1. LysA™ Universal PARylation Assay workflow diagram
In one commercial version of an ELISA PARylation kit, the technique of sandwich ELISA involves coating 96-well plates with a PAR-binding reagent that recognizes PARylated chains. Cell lysates are added to the coated wells, and the antibody captures the PARylated proteins present.
Ultimately, a luminescence signal results that is directly correlated with the amount of PARylation in the cell extracts (Figure 1). The sensitivity is often on the order of 100 pM PAR.8
These kits are a convenient way to measure PAR levels after exposure to a hemotherapy drug, enabling the assessment of drug efficacy. They are also ideal for analyzing the synergistic effects of various treatment combinations within cells. Furthermore, commercial kits can also determine the IC50 levels (the half-maximal inhibitory concentration) of PARP and PARG inhibitors. Finally, PAR levels in biological samples can be quantified using PAR standards.
It is worth noting that some commercial PARylation assay kits are solely designed to detect PARylation. In contrast, they may or may not detect the related process of MARylation (mono ADP-ribosylation), in which a single ADP-ribose unit is added to a protein.9 Therefore, researchers need to assess ahead of time whether a given ELISA assay is suitable for measuring MARylation.
More information about commercial ELISA kits for PARylation analysis can be found here: Total Cellular PARylation: From Inquiry to Insight in Drug Discovery

"Understanding and measuring PARylation is key to advancing cancer research and drug development. ELISA-based methods provide a convenient, reliable approach for researchers to assess PARylation levels in intact cells, making them invaluable tools for exploring therapeutic targets."
-
References
1. Kang M, Park S, Park SH, Lee HG, Park JH. A double edged sword: The two faces of PARylation. Int J Mol Sci. 2022;23(17).
2. Martincuks A, Zhang C, Austria T, Li YJ, Huang R, Lugo Santiago N, et al. Targeting PARG induces tumor cell growth inhibition and antitumor immune response by reducing phosphorylated STAT3 in ovarian cancer. J Immunother Cancer. 2024;12(4).
3. Bhamidipati D, Haro-Silerio JI, Yap TA, Ngoi N. PARP inhibitors: enhancing efficacy through rational combinations. Br J Cancer. 2023;129(6):904-16.
4. Cellular PARylation: From inquiry to insight in drug discovery: BPS Bioscience
5. Yamashita S, Tanaka M, Ida C, Kouyama K, Nakae S, Matsuki T, et al. Physiological levels of poly(ADP ribose) during the cell cycle regulate HeLa cell proliferation. Exp Cell Res. 2022;417(1):113163.
6. Ida C, Yamashita S, Tsukada M, Sato T, Eguchi T, Tanaka M, et al. An enzyme-linked immunosorbent assay based system for determining the physiological level of poly(ADP-ribose) in cultured cells. Anal Biochem. 2016;494:76-81.
7. ADP-Ribosylation cycle inhibitor mix BPS Bioscience
8. Total cellular PARylation: From inquiry to insight BPS Bioscience
9. Measuring cellular PARylation to gain insight into PARP/PARG-targeted drug discovery BPS Bioscience
Ready to take the next step?
Not sure which assay suits your research best? Our scientific team is here to help! From product selection to Contract Research Services, we provide expert guidance tailored to your research project needs.