

Download the Application Note
RNA vaccines have shown their potential during the Covid-19 pandemic, and their promise in cancer treatment is on the horizon.
Our Contract Research Services’s new application note explores the high-quality RNA production and precise, cost-effective testing tools essential for oncology research.
Introduction
RNA vaccines have shown their full potential in the Covid-19 pandemic. The application of these technologies for the treatment of cancers represents a significant promise, but clinical effectiveness remains to be demonstrated [1, 2].
For this purpose, researchers require high quality production of RNA vaccine, and accurate and cost-saving test tools that are adapted to oncology research [3]. To respond to this growing need for RNA, improvements in manufacturing throughput and turnaround time are required.
Tebubio Contract Research Services offer a RNA production service providing µg to mg amounts of custom RNA, to be used for screening purposes in preclinical research (vaccine, therapeutic agent, personalized medicine, etc) for the pharmaceutical industries, biotechs and academics.
Construction Design
We synthesized RNAs using our proprietary plasmid DNA templates for linear and circular RNAs with UTRs and poly-A tail optimized for efficient transcription and translation in mammalian cells. The T7-FlashScribe transcription kit (Cellscript, Ref. C-ASF3507) was used for the RNA synthesis.
To test the transfection efficiency, we prepared fluorescent eGFP RNAs by replacing UTPs with Andy Fluor 647-X-UTP probe (AF647, Ref. C418-T, ex/em = 650/665 nm). Transfection in cells was done using the mRNA Fect reagent (RJH Biosciences, Ref. 80-40).
 |
 |
 |
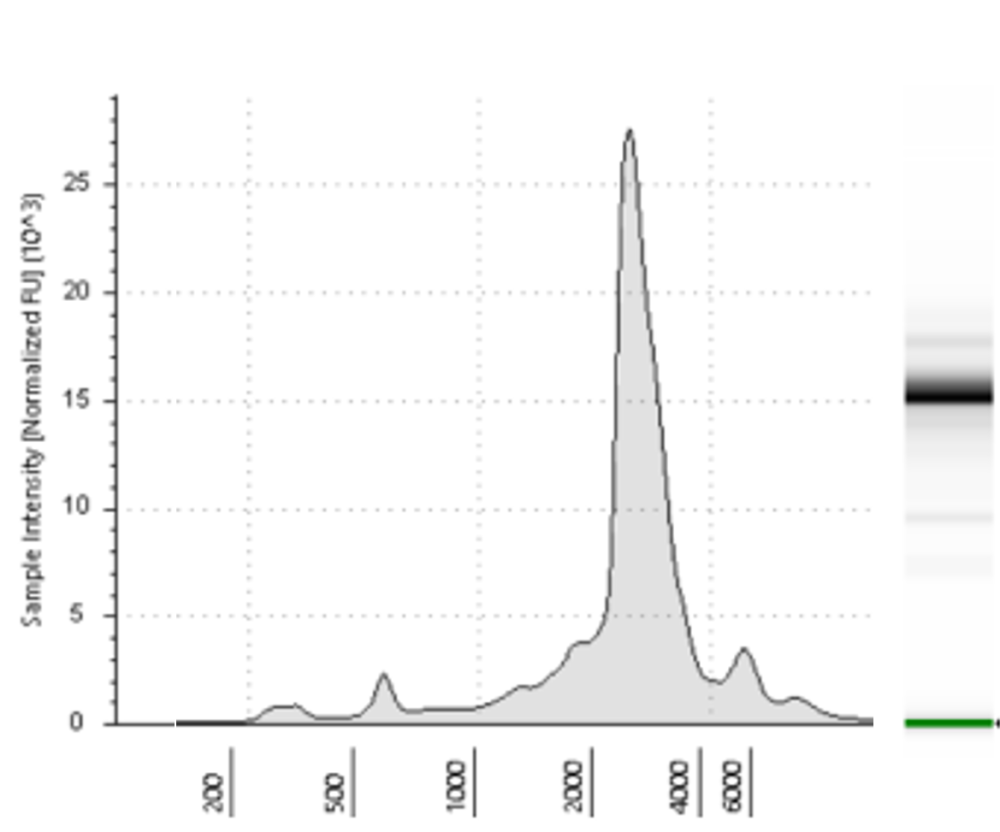 |
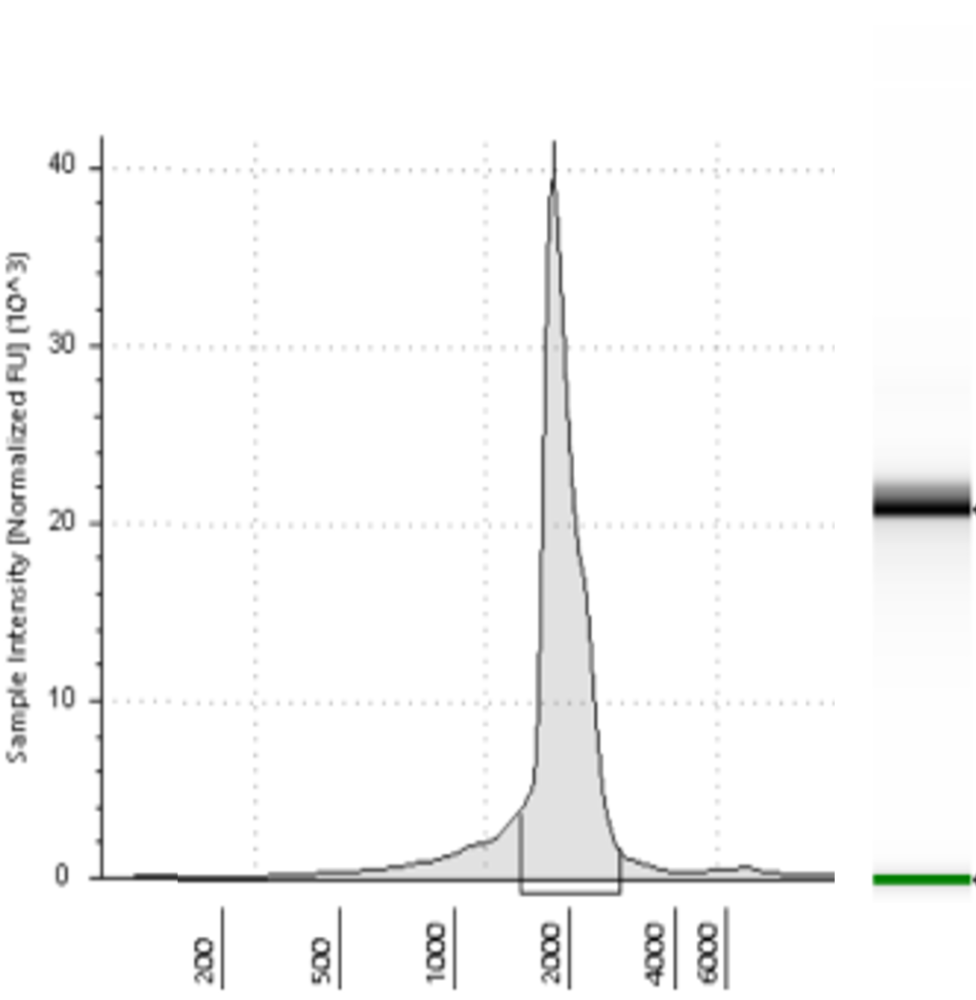
Figure 1. RNA production steps for linear (left panel) and circular (right panel) RNAs. For QC step includes an electrophoresis and its densitometry profile.
eGFP & p53-eGFP expression in HEK293: Linear vs. Circular RNA
The RNAs were transfected into HEK293 cells and the fluorescence was checked by microscopy 24 hours after transfection.
- The level of transfected RNA within HEK293 cells is similar between circular and linear RNA.
- Protein expression is extremely low when transfecting with circular RNA as compared to linear RNA.
- p53-eGFP is expressed in the nucleus and the cytoplasm as expected
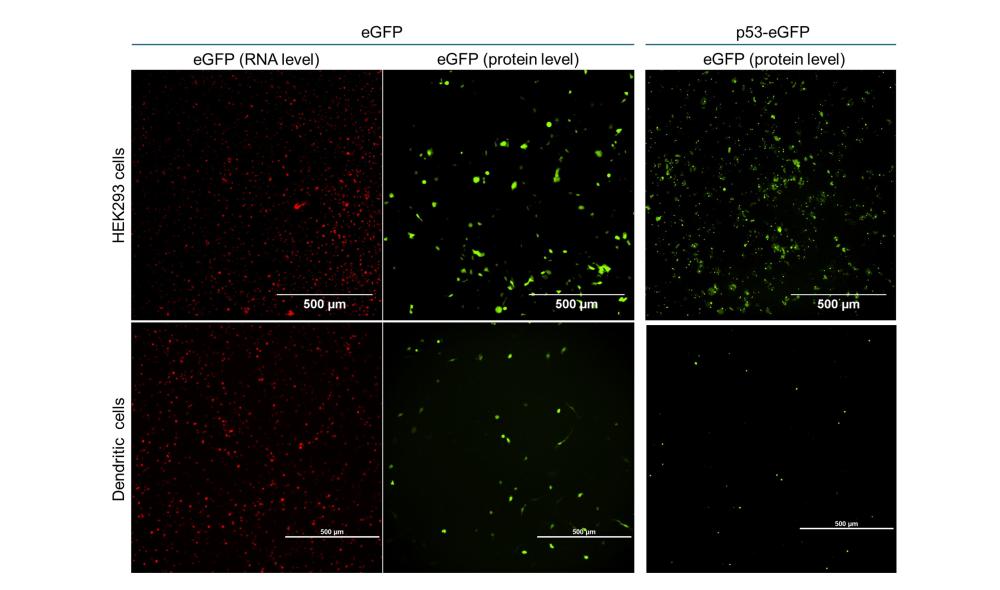 Figure 2. Representative images of HEK293 cells 24 hours after transfection with fluorescent eGFP RNA or p53-eGFP RNA (n=3). Scale bar = 500 µm.
Figure 2. Representative images of HEK293 cells 24 hours after transfection with fluorescent eGFP RNA or p53-eGFP RNA (n=3). Scale bar = 500 µm.
Validation of eGFP RNA expression and stability in APCs (antigen presenting cells)
RNAs were transfected in dendritic cells (Zen-Bio, ref. SER-MODC-F) and the fluorescence was visualized by microscopy at different time points after transfection. We compared eGFP RNAs expression with their fluorescent counterpart.
- Global RNAs expression at the protein level is weaker in APCs than in HEK293 model.
- There is no eGFP expression using circular RNAs in APCs model (Table 1 & Figure 3).
- Presence of fluorescent UTP decreases the final expression capacity (Table 1 & Figure 3).
- eGFP protein is detected from 5h to 5 days post-transfection. A peak of protein of expression is observed between 24 and 48h after transfection (Table 1).
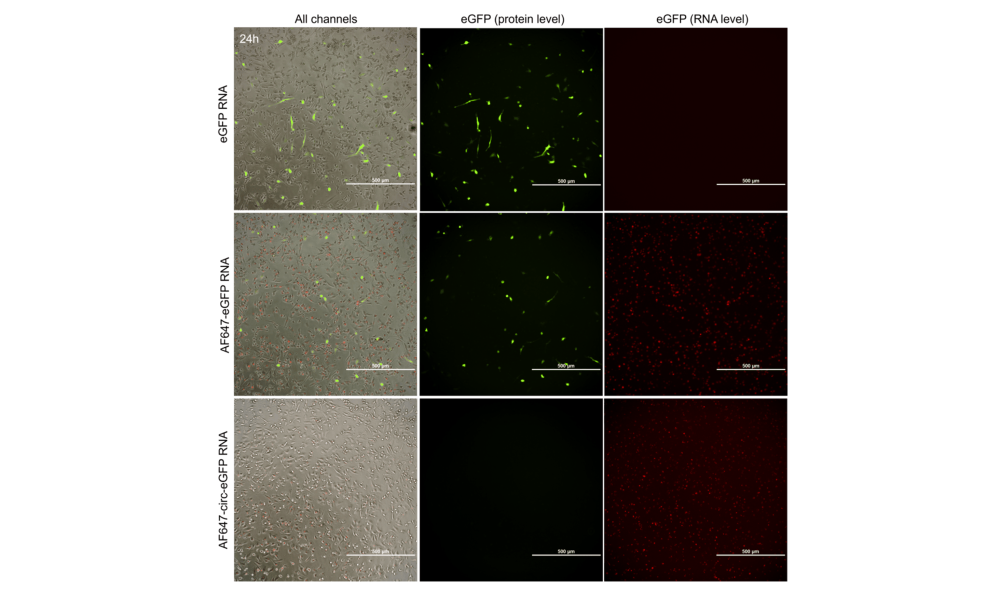
Figure 3. Representative images of dendritic cells transfected with linear eGFP or fluorescent linear and circular eGFP RNAs at 24h post-transfection (n=3). Scale bar=500 µm.
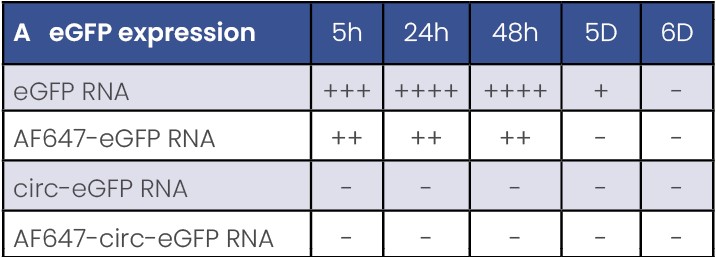
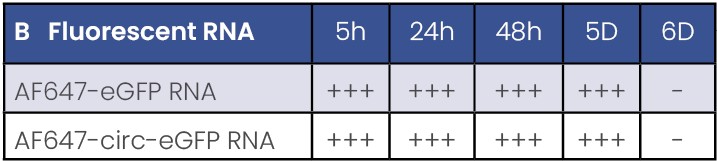
Table 1. A: levels of eGFP protein expression after RNA transfection in dendritic cells.
B: levels of fluorescent RNA (AF647) after transfection in dendritic cells.
p53 expression and cytokine release in APCs
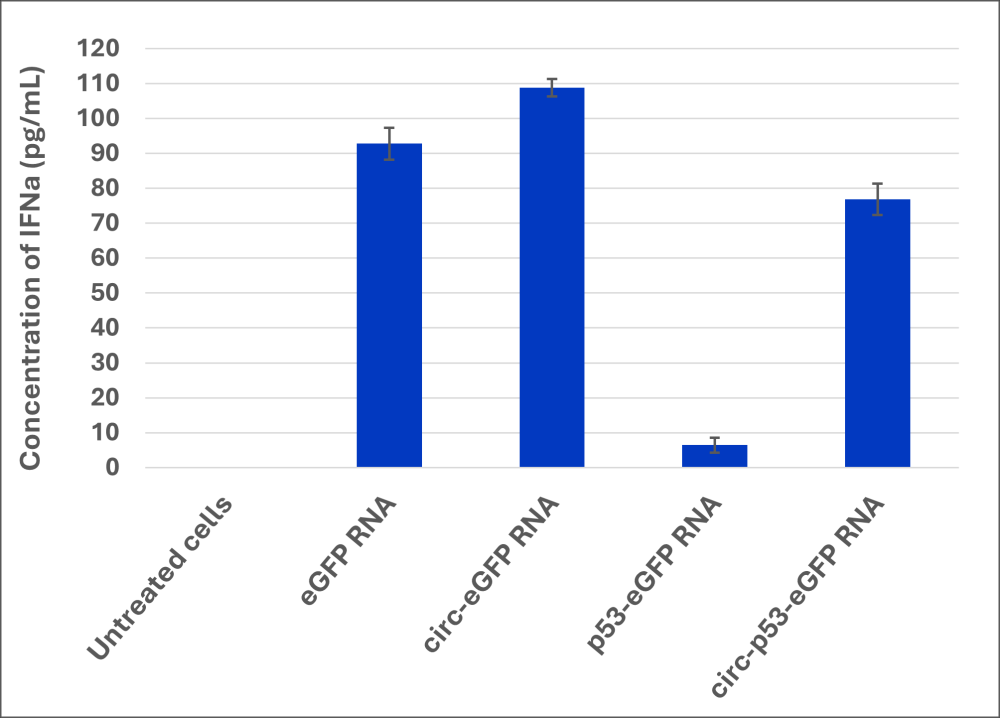
RNAs were transfected in dendritic cells (Zen-Bio, ref. SER-MODC-F). 48h post-transfection, p53 expression was checked by immunofluorescent (IF) staining (Figure 4). Media were collected 48h after transfection to quantify the cytokine release by multiplex ELISA (Q-Plex™ Human Innate Immunity, Quansys Biosciences, ref. 111233HU) for the measurement of GMCSF, IFNα, IFNβ, IFNγ, IL-1α, IL-1β, IL-18 (Figure 5).
- eGFP is expressed in the whole cells while p53-eGFP or p53 are mainly located in nucleus. (Figure 4)
- Multiplex analysis enabled the quantification of IFNα and IL-18 released by dendritic cells. (Figure 5)
- IFNα secretion in the medium is strongly promoted in the cells transfected with linear and circular eGFP RNAs and circular p53-eGFP RNA while it is slightly increased in cells transfected with linear p53-eGFP RNA as compared to control cells.
- We observe a similar pattern of release with IL-18.
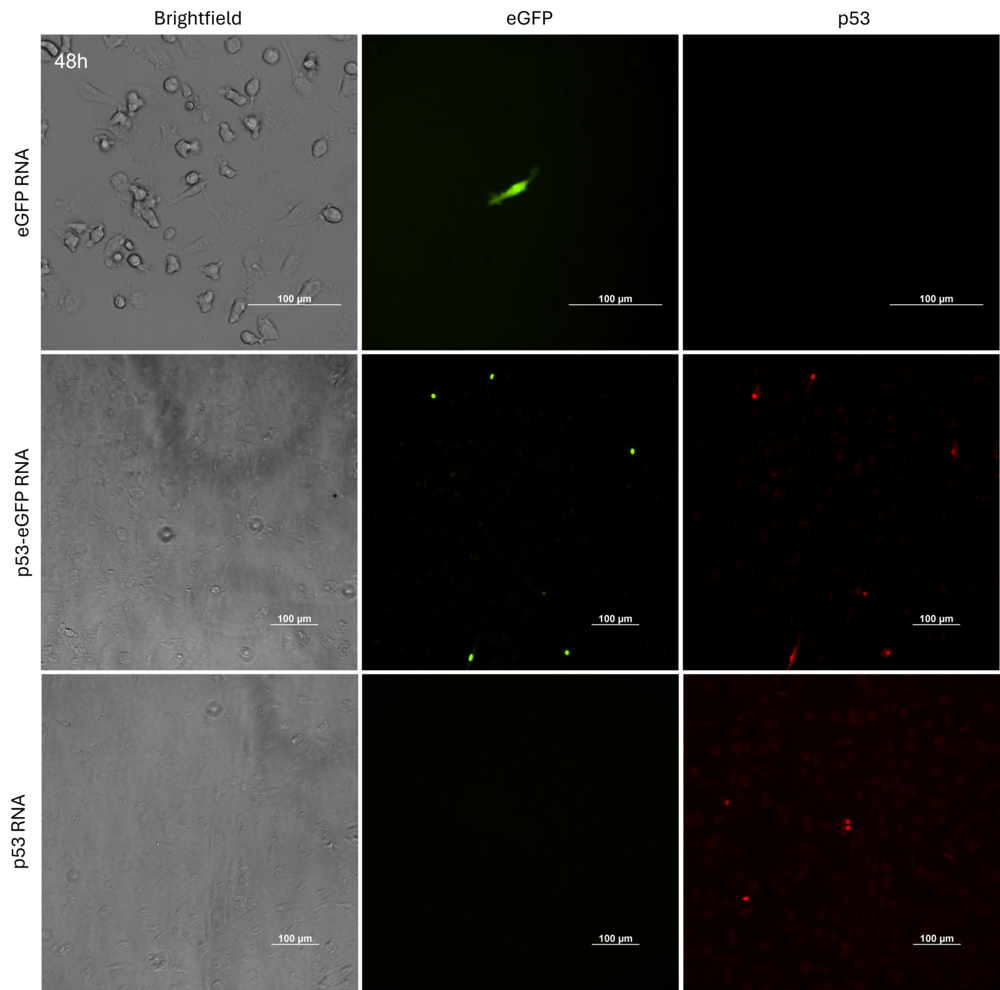
Figure 4. Immunofluorescent staining in dendritic cells transfected with eGFP, p53, and p53-eGFP linear RNAs (n=3). The cells were fixed in 4% paraformaldehyde and p53 stained by p53 Polyclonal Antibody (ThermoFisher Scientific, ref. PA5=27822). Scale bar = 100 µm.
 |
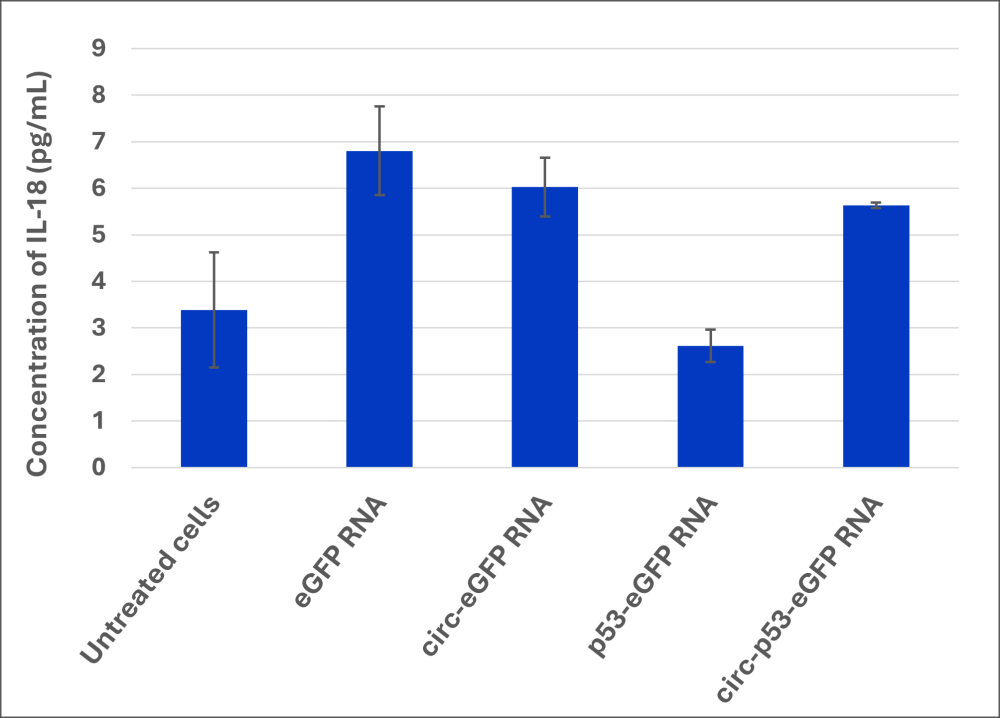 |
Figure 5. Multiplex quantification of cytokines IFNα and IL-18 released by dendritic cells (n=3)
Conclusion
- Circular RNA is hardly expressed in both HEK293 and APC models.
- p53 expression leads to the modulation of the release of pro-inflammatory cytokine in vitro.
- Anti-tumoral antigen expression using Linear RNA is a promising technology for lymphocytes activation against tumor cells.
- Transfection using custom LNP fomulation is the next step managed by Tebubio Contract Research Services team.
Realised with the support of:
![]()

-
Authors
Célia Bosso-Lefèvre, PhD - Erica Cirri, PhD - Eric Mennesson, PhD - Dimitri Szymczak, PhD - Flavien Carpentier - Alexandra Foucher - Alzbeta Komarkova - Maxime Rochet - Armelle Vindrios - Romain Goulay-Cordonnier, PhD - Jean-François Têtu.
-
Affiliation
Tebubio - 39 rue de Houdan, Le Perray en Yvelines, France
Optimize Your RNA Synthesis Process
We can propose tailored solutions designed to enhance the efficiency and scalability of your RNA-based vaccine manufacturing.

