In this post, I'd like to introduce the Human liver Lysosomes developed by Sekisui-Xenotech, which have opened up a new era in catabolism models. Read on to learn more, and also to download a case study demonstrating the use of lysosomes for catabolism of human IgG.
Lysosomes are membrane-bound cellular organelles. They are the site of degradation/catabolism, including extracellular substrates (endocytic pathway) and intracellular substrates (autophagy pathway). Lysosomes contain a multitude of acidic hydrolytic enzymes. They vary greatly in size and have Isopycnic densities similar to mitochondria.
Considering their unique pH and the presence of a variety of catabolic enzymes, therapeutic strategies are being designed to take advantage of lysosomes as the primary site of catabolism/activation for targeted biopharmaceuticals that enter cells through the endosomal-lysosomal pathway.
Why use Human Liver Lysosomes in in vitro drug development?
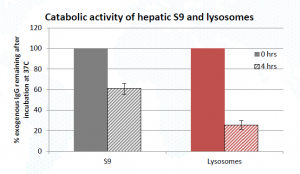
Sekisui XenoTech’s Human Liver Lysosomes provide the most high-quality, specific, relevant, easy and ready to use in vitro test system to monitor stability/catabolism of targeted biotherapeutics. Purified recombinant enzymes, cell homogenates or cell lines engineered to overexpress certain proteins cannot replicate the environment that compounds will encounter in the native lysosome, nor do they take into account inherent differences between primary human tissue and cell lines.
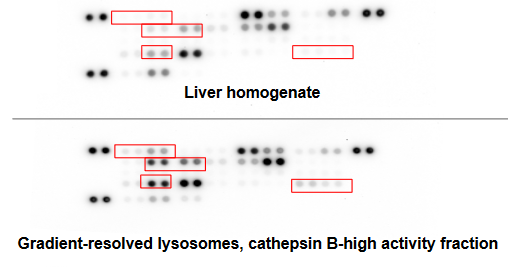
Purified hepatic lysosomes offer a complete and stoichiometrically correct enrichment of lysosomal contents from human primary tissue for greater lysosomal enzymatic activity and enrichment of lysosomal proteins with low contaminating activity from mitochondrial enzymes. The lysosomal isolation protocol used has been optimized through characterization by immunoblot, enzymatic activity and protease content.
Applications of the Human Liver Lysosomes
Lysosomes can be used as an in vitro diagnostic tool to conveniently, cost-effectively and quickly evaluate potential changes in lysosomal stability due to targeted modifications of the biopharmaceutical/macromolecule during development. The data can help narrow and direct development tracks of biopharmaceuticals such as those interested in:
- Antibody-Drug-Conjugates (ADCs)
- siRNA/RNAi technologies
- immunotherapies
- biodegradable copolymers and nanoparticles as delivery mechanisms
- cosmetics using microparticles etc.
Case study with Human Liver Lysosomes: Functional characterization – catabolism of human IgG
Also available: Rat Tritosomes
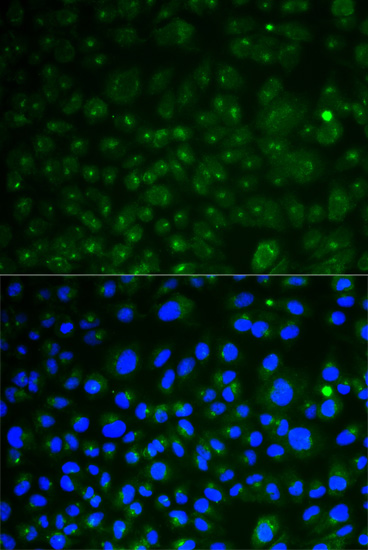 IF HeLa cells with anti CD107a polyclonal antibody Cat. nr 281BS6978 Bioworld | tebu-bio.
IF HeLa cells with anti CD107a polyclonal antibody Cat. nr 281BS6978 Bioworld | tebu-bio.Tritosomes are lysosomes isolated from Tyloxapol-treated animals for improved separation of the organelle from the mitochondria.
- Cathepsin L Human ELISA Kit - cat. nr 126ELH-CathepsinL
- Cathepsin S Human ELISA Kit - cat. nr 126ELH-CathepsinS
- Cathepsin B Activity Assay Kit - 12668AT-CathB-S100
- Acid Phosphatase Activity Colorimetric Activity Assay Kit - 12668CL-AcPh-S500
- Anti LAMP-1 / CD107a polyclonal antibody - cat. nr 281BS6978
- Anti CTSD monoclonal antibody (M01), clone 3F12-1B9 - cat. nr 157H00001509-M01
- Bafilomycin A1 | Vacuolar H+ ATPase inhibitor (CAS: 88899-55-2 - cat. nr 21910-2060)
Bafilomycin A1 (macrolide antibiotic) inhibits autophagy via disruption of fusion between autophagosomes and lysosomes.