

Download the Application Note
Our Contract Research Services (CRS) team developed eGFP mRNA labeled with a fluorophore to monitor its delivery and expression efficiency in cells.
Introduction
The application of mRNA in therapeutics, diagnostics and analytical methods has significantly increased in recent years. To answer the growing need for mRNA, improvements in manufacturing throughput and turnaround time are required.
Tebubio laboratories offer an mRNA production service providing µg amounts of custom mRNA in as little as 5 days, to be used for screening purposes in preclinical research (vaccine, therapeutic agent, personalized medicine, etc) for the pharmaceutical industry, biotechs and academics.
In this context, our laboratory developed eGFP mRNA labeled with a fluorophore to monitor its delivery and expression efficiency in cells. The same labeling approach can be also applied to any mRNA for clients who wish to monitor its delivery in cells or encapsulation in LNPs
Materials & Methods
We synthesized RNA using our proprietary plasmid DNA template with UTRs and poly-A tail optimized for efficient transcription and translation in mammalian cells. The T7-FlashScribe transcription kit (Ref. C-ASF3507) was used for the mRNA synthesis.
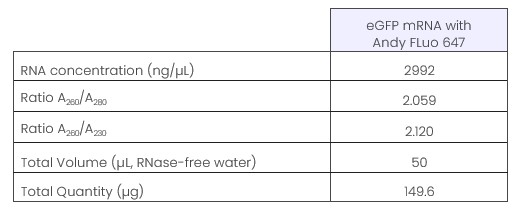
In order to test the efficiency of incorporation of nucleotides into mRNA during in vitro transcription as well as their effect on protein translation, we prepared eGFP mRNA by replacing a part of the unmodified UTP in the transcription reaction with Andy Fluor 647 -X-UTP probe (Ref. C418-T), characterized by the wavelengths ex/em = 650/665 nm. Analysis of the generated mRNA was carried out by NanoVue Plus (GE Healthcare) as well as by
capillary electrophoresis with TapeStation 4150 (Agilent).
After that, the mRNA was transfected into HEK293 cells with the mRNA-Fect reagent (Ref. 80-40), and the fluorescence was checked by microscopy 24 hours after transfection, following the washing step with PBS to eliminate the non-transfected mRNA
Results
To verify properties of the mRNA, we produced labeled and unlabeled eGFP mRNA. The mRNA of interest showed
equivalent yield and quality to unmodified mRNA (Figure 1).
a)

b)
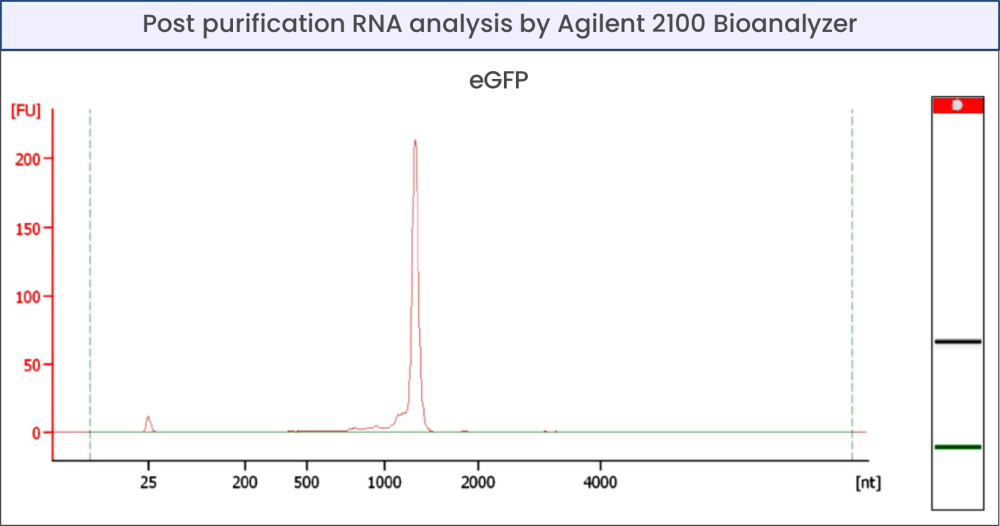
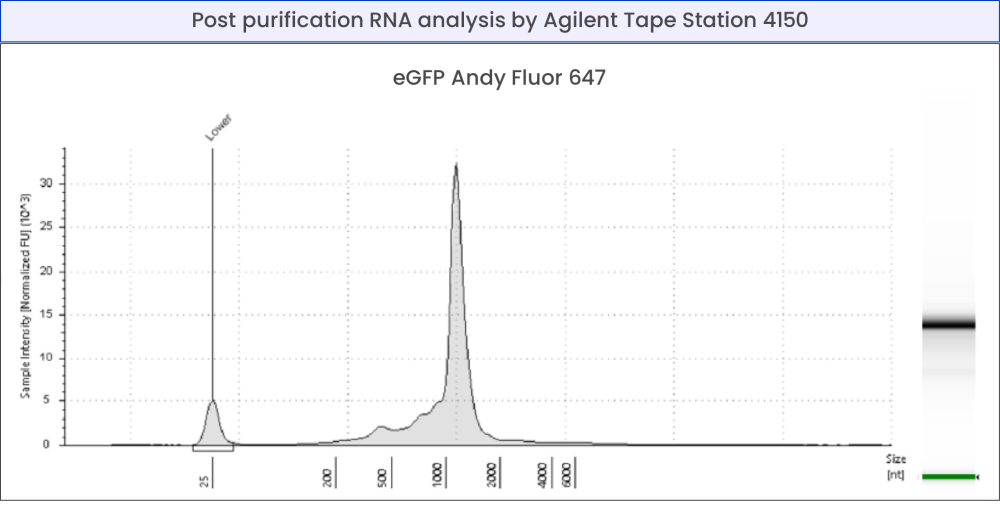
Figure 1 - Production of unlabeled eGFP mRNA and labeled with Andy Fluor 647
Quality control of mRNA by analysis with NanoVue Plus (GE Healthcare) (a) and TapeStation 4150 (Agilent) (b).
We then verified the delivery efficiency of the labeled mRNA and the expression efficiency in HEK293 cells by fluorescence microscopy, which was performed 24 hours after transfection, and cells were followed by a washing step with PBS.
 Figure 2 - Transfection of HEK293 cells with Andy Fluor 647-labeled eGFP mRNA
Figure 2 - Transfection of HEK293 cells with Andy Fluor 647-labeled eGFP mRNA
Left - Detection of fluorescent eGFP mRNA by fluorescence microscopy using Cy5 (665 nm) filter
Centre - Detection of eGFP protein expression by fluorescence microscopy using FITC (510 nm) filter
Right - Bright field of HEK293 Cells
All results indicate that the fluorophore is sufficiently stable to allow the detection of mRNA in cells which are both transfected and translated efficiently despite the presence of the label.
Conclusion
After the Incorporation of fluorescent UTP into the mRNA, we can see that yield and quality are similar with and without fluorescent UTP. Following the delivery of mRNA into HEK293 cells, the presence of the fluorophore allows the visualization of the transfected mRNA in cells.
The result also proved that the labeled eGFP mRNA produced this time was translated efficiently, even with the presence of fluo UTP.
Realised with the support of:
![]()

-
Authors
Erica Cirri, PhD - Maxime Rochet - Alexandra Foucher - Alzbeta Komakova - Xiyu Qian
-
Affiliation
Tebubio - 39 rue de Houdan, Le Perray en Yvelines, France
Need Laboratory Services? Get a Personalized Quote Today!
Leverage our expert laboratory services for your RNA production needs. Contact us for a tailored solution and quotation.

